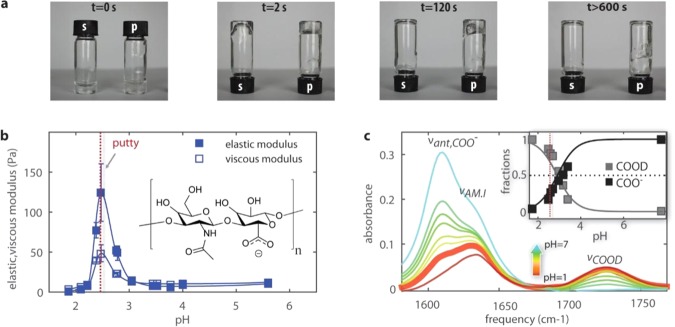
Figure 1.
Hyaluronic acid solutions undergo a sol–gel transition in a narrow pH range. (a) A tube inversion assay shows that hyaluronic acid solutions prepared at pH 1.6 exhibit viscous flow (s), whereas solutions prepared at pH 2.5 form a viscoelastic gel (p) that flows only on time scales beyond 2 min. (b) The pH dependence of the viscous and elastic modulus of hyaluronic acid in heavy water solutions. We observe a sharp peak in the elastic shear modulus at pH 2.5. The inset shows that hyaluronic acid is a polymer of disaccharides, themselves composed of d-glucuronic acid and N-acetyl-d-glucosamine. (c) Linear infrared spectra for hyaluronic acid solutions in D2O at pH values ranging between 1.6 and 7. The infrared spectrum at pH = 2.5 is represented by the thick solid line. Between 1550 and 1760 cm–1, we observe three bands: the antisymmetric stretching mode of the carboxylate anion group, νant,COO–, at 1607 cm–1, the amide I vibration, νAM.I, at 1633 cm–1, and the carboxylic acid stretching mode, νCOOD, at 1726 cm–1. The inset shows the fractions of COO– and COOD groups determined from the IR spectra as a function of pH. Thick lines represent the expected fractions based on acid–base equilibrium equations (Supporting Information), assuming a pKa = 2.9. We find good agreement between the measured and expected carboxylic and carboxylate fractions, with about 25% deprotonation of the carboxyl groups at pH = 2.5.