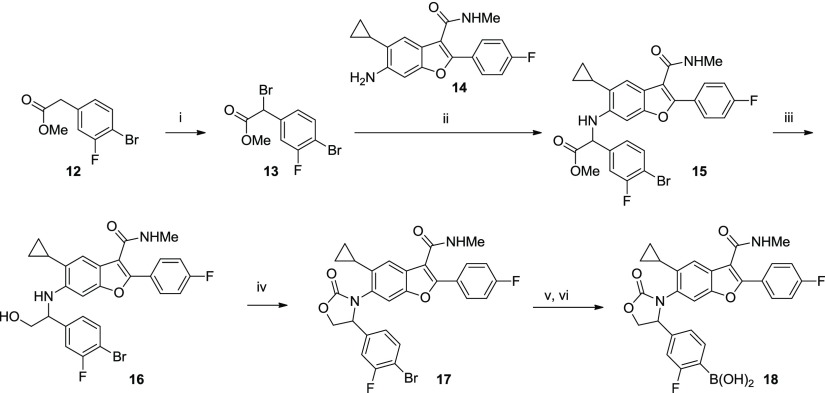
Scheme 1. Representative Synthesis of Oxazolidinone 18.
Conditions: (i) NBS, CCl4, benzoyl peroxide, reflux, 4 h, 71%; (ii) aniline 14, DMF, 85 °C, 47%; (iii) LiBH4, THF, 84%; (iv) triphosgene, Et3N, DCM, 50%; (v) (BPin)2, PdCl2(dppf)·CH2Cl2, KOAc, dioxane, 100 °C; and (vi) PS-BBA, HCl, THF, 30% for 2 steps.