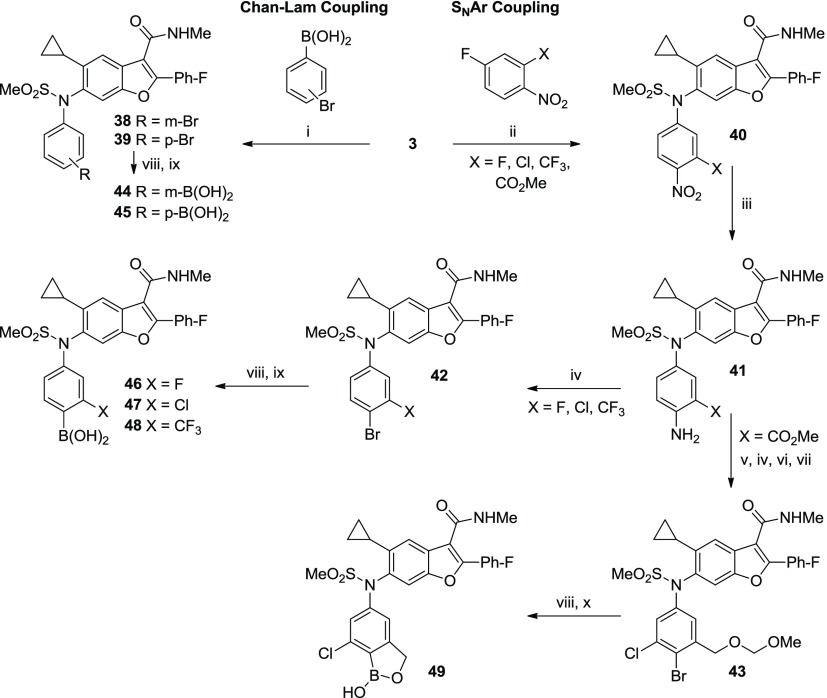
Scheme 3. Synthesis of N-Phenyl Boronic Acid Analogs (44–49).
Conditions: (i) Cu(OAc)2·H2O, TEA, 4 Å mol. sieves, DCM, 26–39%; (ii) K2CO3, DME, H2O, 80–100 °C or K2CO3, HMPA, 60 °C, 29–93%; (iii) SnCl2 or H2 with Pd/C or sulfided Pt/C, 50–100%; (iv) NaNO2, CuBr, HBr, MeCN, H2O, 0–60 °C, 73–76%; (v) NCS, DMF, 60 °C, 99%; (vi) LiBH4, THF, MeOH, 5 °C, 100%; (vii) MOMCl, DIEA, THF, 50 °C, 68%; (viii) (BPin)2, PdCl2(dppf)-CH2Cl2 or Pd(dppb)Cl2, KOAc, dioxane, 80 °C; (ix) PS-BBA, HCl, H2O, THF or NaIO4, HCl, H2O, THF, 15–74% (2 steps); and (x) HCl, H2O, THF, MeOH, 71% (2 steps).