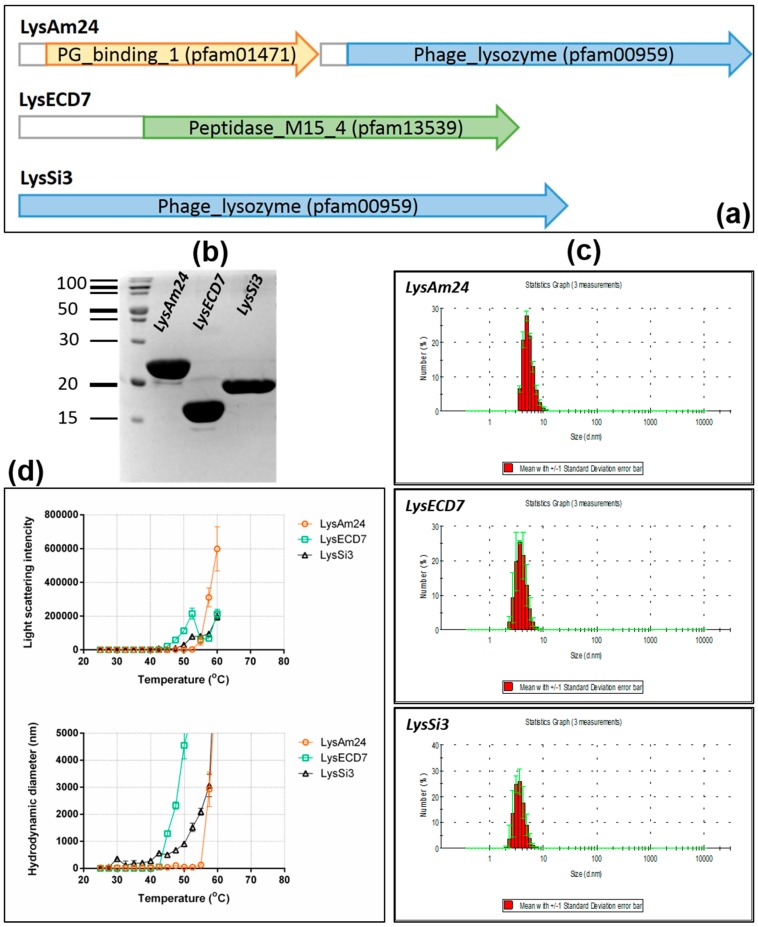
Figure 1.
Domain organization, purification, and physicochemical properties of LysAm24, LysECD7, and LysSi3. (a) Putative domain organization of LysAm24, LysECD7, and LysSi3 predicted from the deduced amino acid sequences. The prediction was done with the protein BLAST search (https://blast.ncbi.nlm.nih.gov/Blast.cgi). (b) SDS-PAGE gel analysis of purified endolysins. The PageRuler Broad range Unstained Protein Ladder (Thermo Scientific, Vilnius, Lithuania) was used (c) Evaluation of the hydrodynamic diameter of E. coli-produced endolysins by DLS analysis. Statistical distribution of particle size by number. The data are presented as the mean values of three measurements ± SD. (d) Temperature dependence of the total light scattering intensity (upper panel) and the hydrodynamic diameters of the particles (lower panel). The data are presented as the mean values of three measurements made with an interval of 15 s ± SD.