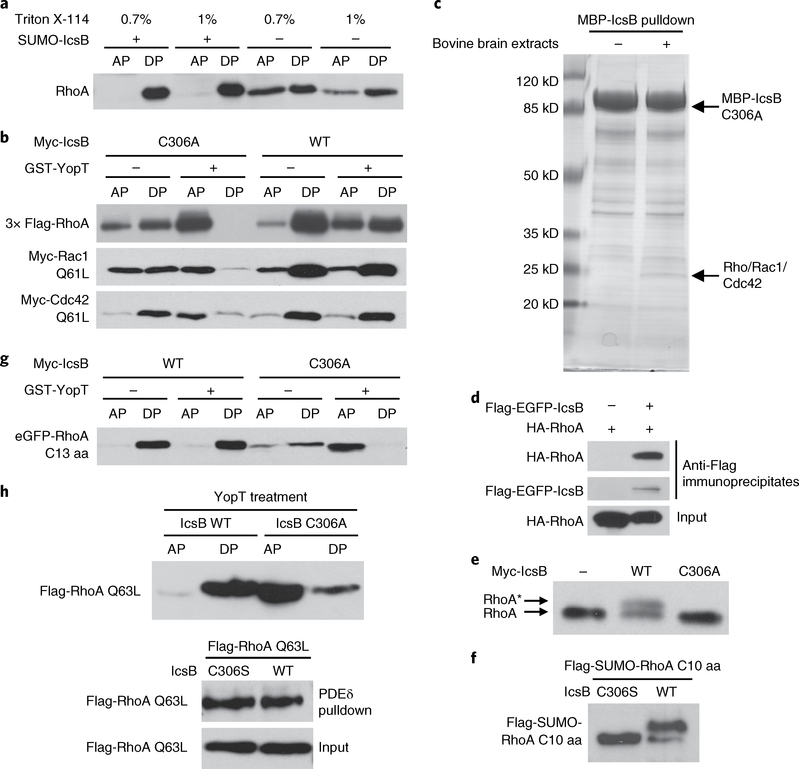
Fig. 2 |. IcsB disrupts RhogTPase membrane cycling by modifying its C-terminal tail.
a, Effects of IcsB on the hydrophobicity of RhoA. Cytosolic extracts of RhoA-transfected 293T cells were incubated with purified IcsB and ATP followed by Triton X-114 partitioning. b, Effects of IcsB on RhoGTPase cleavage by YopT. 293T cells were co-transfected with an indicated Rho expression plasmid and IcsB (WT or C306A). Cell lysates were treated with or without YopT, then subjected to Triton X-114 partitioning. c, Identification of RhoGTPases as IcsB-binding proteins. Purified MBP-IcsB C306A was incubated with or without bovine brain extracts and then subjected to amylose bead pulldown. Bound proteins were separated on the SDS-PAGE gel and Coomassie blue staining of the gel is shown. The specific band bound by IcsB C306A contains Rho, Rac and Cdc42, revealed by mass spectrometry. d, Interaction between transfected IcsB and RhoA in 293T cells. Shown are immunoblots of anti-Flag immunoprecipitates and total cell lysates (input). e,f, SDS-PAGE mobility shift of the endogenous RhoA or RhoA C-terminal tail induced by IcsB. 293T cells were transfected with an indicated IcsB expression plasmid alone (e) or co-transfected with a Flag-SUMO-tagged RhoA C-terminal ten-residue tail (C10 aa) (f). Cell lysates were subjected to 15% SDS-PAGE, followed by immunoblotting analyses. The asterisk in (e) indicates the mobility shift of the endogenous RhoA. g, Effects of IcsB on the cleavage of the RhoA C-terminal tail by YopT. 293T cells were co-transfected with an eGFP-tagged RhoA C-terminal 13-residue tail (C13 aa) and IcsB (WT or C306A). Cell lysates were treated with or without YopT, then subjected to Triton X-114 partitioning. h, Effects of IcsB on RhoA precipitation by PDEδ. 293T cells were co-transfected with Flag-RhoA Q63L and an indicated IcsB expression plasmid. Cell lysates were subjected to GST-PDEδ pulldown or YopT digestion followed by Triton X-114 partitioning. Proteins in the aqueous phase (AP) and detergent phase (DP) were subjected to immunoblotting analyses (a, b, g and h). All data (a–h) are representative of three independent experiments.