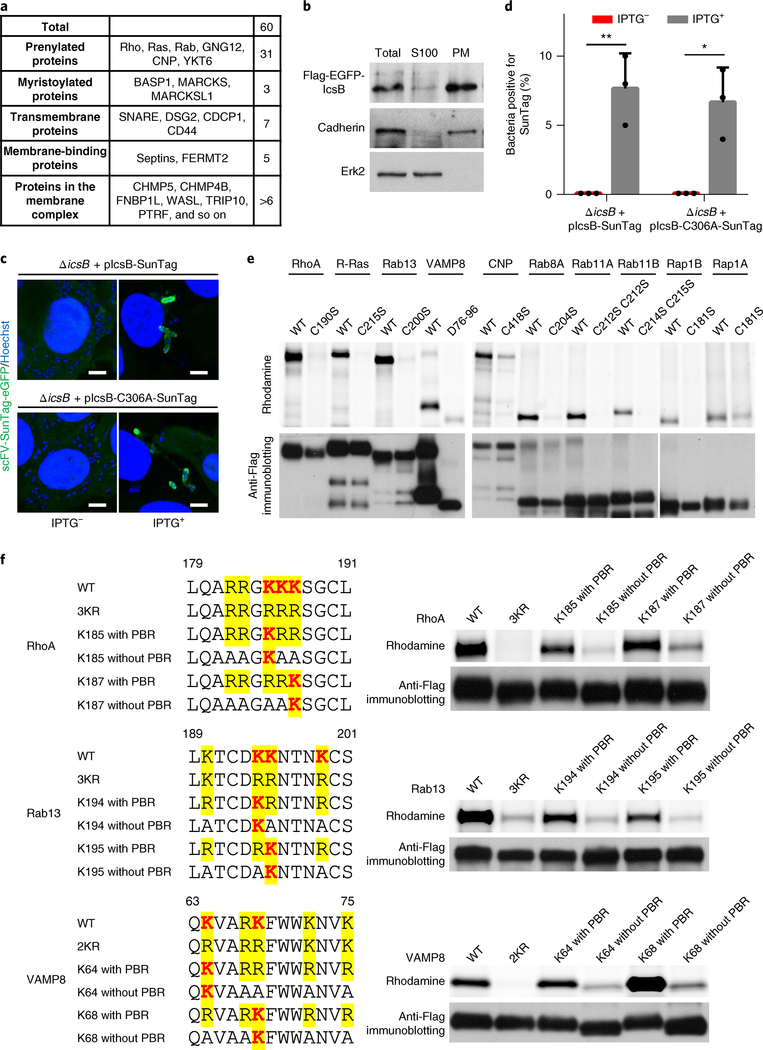
Fig. 5 |. IcsB is localized on Shigella-containing vacuoles and modifies its substrates on the membrane location.
a, Classification of IcsB substrates by their membrane-targeting mechanisms. b, Sub-cellular fractionation of IcsB. 293T cells transfected with Flag-eGFP-IcsB were lysed in hypotonic buffer, and the cell lysates (S10) were ultracentrifuged (100,000g) to obtain the plasma membrane (PM) and the S100 cytoplasmic fraction. Shown are the anti-Flag, anti-cadherin and anti-Erk2 immunoblots. c,d, Localization of IcsB during S. flexneri infection. HeLa cells stably expressing scFV-SunTag-eGFP were infected with S. flexneri Δ icsB complemented with 24× SunTag-fused IcsB (WT or C306A). IPTG was used to induce IcsB expression. Fluorescence images taken at 4 h post-infection are shown in c (scale bars, 3 μm), and percentages of intracellular bacteria containing SunTag-positive signals are shown in d. At least 200 infected cells were examined for each experiment, and data are mean ± s.d. from three replicates. A two-tailed unpaired Student’s t-test was performed (*P < 0.05; **P < 0.01). e,f, Membrane localization of the substrates is required for their modification by IcsB. 293T cells were co-transfected with IcsB and an indicated Flag-tagged substrate. The mutants assayed in e are cysteine mutants devoid of lipid modification or deletion of the transmembrane helix for VAMP8 (D76–96). f, RhoA, Rab13 and VAMP8 mutants with or without the PBR, both of which bear a single modifiable lysine, were assayed. Cells were metabolized with Alk-16 and harvested for in-gel fluorescence assay. Data (b–f) are representative of three independent experiments.