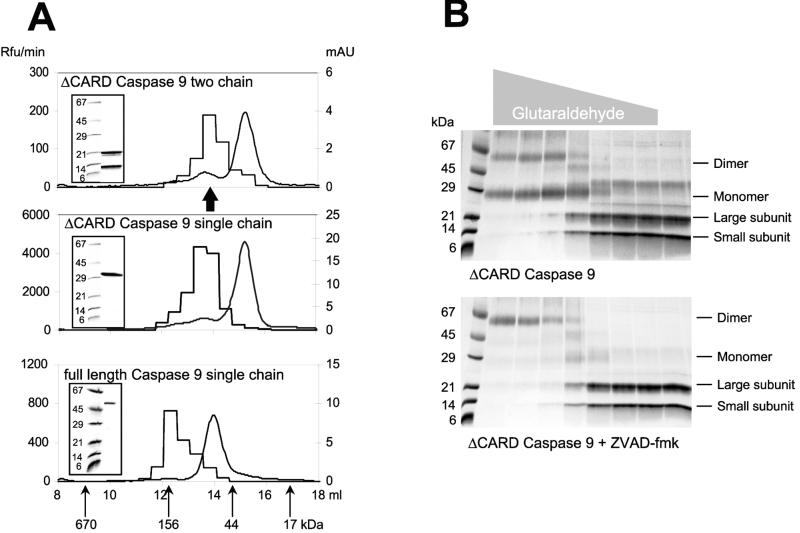
Figure 1.
Identification of the active form of caspase 9. (A) Gel filtration of caspase 9 on a Amersham Pharmacia Superdex 200 column reveals that most of the protein for each form (smooth curge; right ordinate, mAU = milli-absorbance units at 280 nm) eluted as a monomer (≈35 kDa for the ΔCARD forms and ≈48 kDa for the full-length form), but the majority of activity against the substrate Ac-LEHD-AFC (step curve; left ordinate, Rfu = relative fluorescence units) is present in the fraction corresponding to dimers (≈70 kDa for the ΔCARD forms and ≈100 kDa for the full length form). Insets demonstrate the integrity of the purified recombinant proteins run in SDS/PAGE before gel filtration. The arrow marks the position of the protein peak for two chain caspase 9 inhibited by Z-VAD-FMK. (B) Crosslinking of ΔCARD caspase 9 by glutaraldehyde in the absence (Upper) or presence (Lower) of 1.0 μM Z-VAD-FMK. The gray triangle above the gels indicates the glutaraldehyde concentration (ranging from 500 to 0.032 mM). The majority of caspase 9 material is a monomer, and inhibitor binding forces the dimeric form. Thus, it appears that the inhibitor either drives or traps the dimeric form of caspase 9, indicating that the dimer is the active form of the enzyme.