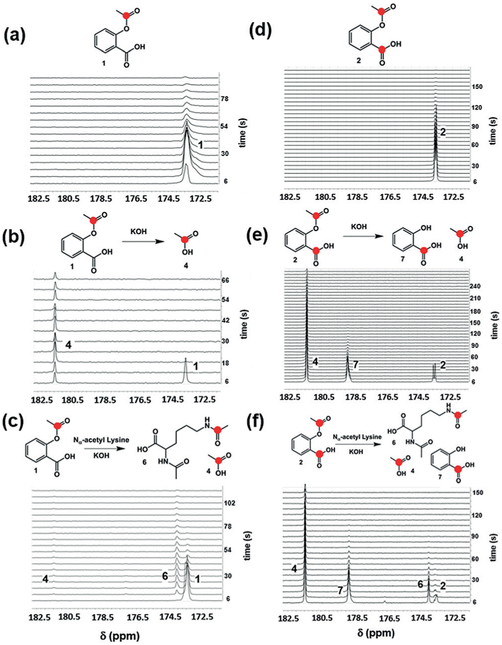
Figure 2.
In vitro reactions with hyperpolarized 1 and 2. a) Hyperpolarized 1 in phosphate-buffered saline (PBS, 100% D2O, 7 T). b) Hyperpolarized 1 in 1 m KOH hydrolyzes rapidly to form hyperpolarized acetate 4, seen at 181.5 ppm. c) Hyperpolarized 1 in 1 m KOH and 250 mM Nα-acetyl lysine undergoes rapid transacetylation to form Nα, 13C-Nε-diacetyl lysine 6, seen at 174.1 ppm. d) Hyperpolarized 2 in PBS (100% D2O). Both 13C-labeled carbons were observed as a partially resolved doublet at 173.3–173.6 ppm (7 T). e) Hydrolysis of hyperpolarized 2 in excess KOH resulted in the formation of 13C-acetate 4 and 13C-salicylic acid 7 (178.7 ppm). f) Reaction with hyperpolarized 2 and Nα-acetyl lysine showed the expected Nα, 13C-Nε-diacetyl lysine 6 as well as 13C-acetate and 13C-salicylic acid.