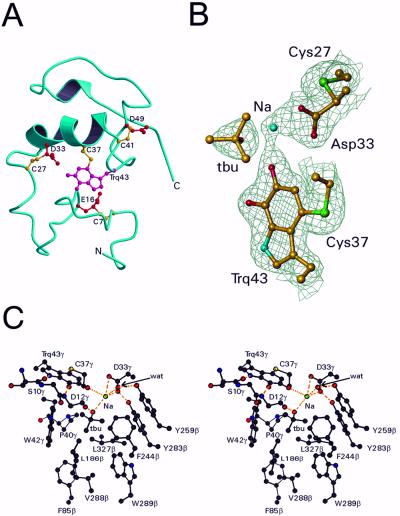
Figure 3.
Structure of the γ subunit and active site of QHNDH. (A) The backbone ribbon is blue, the crosslinked side chains of Cys are yellow, and the crosslinked side chains of tryptophylquinone (Trq), Glu, and Asp are red. (B) Electron density around CTQ. Also shown are the putative active site base Asp-33γ, its covalently linked Cys-27γ, the molecule of t-butyl alcohol (tbu) and the sodium ion (Na). Only Cα and side chain atoms are included. The map was computed with coefficients (2Fo − Fc) exp(−iα) where Fc and α, the calculated structure factors and phase angles, were derived from the final refined model. The contours are drawn at 1.2 σ, where σ is the rms value of the electron density. This diagram was prepared by using TURBO-FRODO (18). (C) Stereoview of the active site of QHNDH. See text for details.