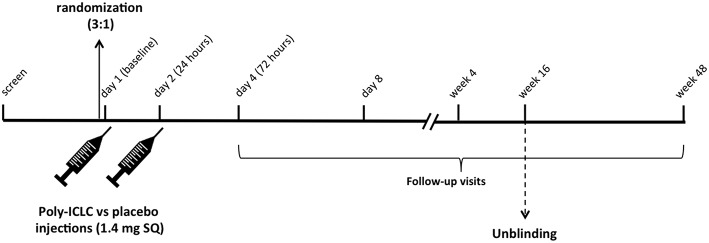
Figure 1.
Study Schema. Following screening, eligible subjects are randomized 3:1 to receive Poly-ICLC (1.4 mg SQ) vs. Placebo on days 1 and 2. Participants returned for follow up visits on days 4 and 8, weeks 4, 16, and 48. Unblinding of the study occurred after all subjects completed the week 16 follow up visit as specified by the protocol.