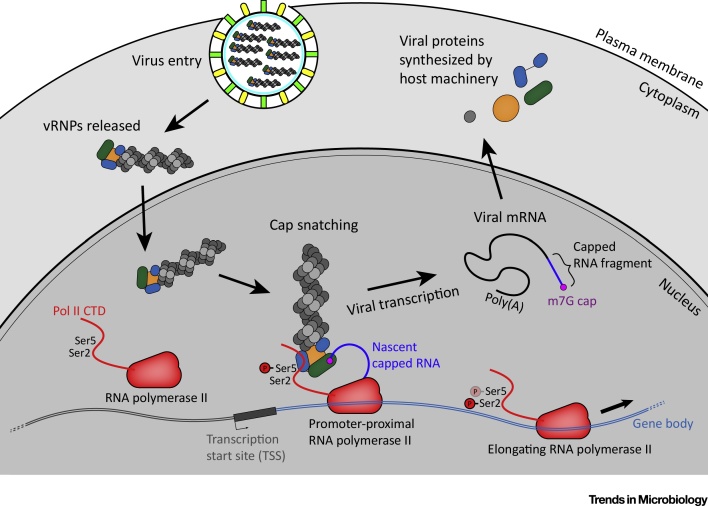
Figure 1.
The Association of Influenza vRNPs with Cellular Pol II. Influenza virions bind to receptors at the plasma membrane and are endocytosed. Viral and endosomal membranes fuse, releasing vRNPs into the cytoplasm. vRNPs are then trafficked to the nucleus to target cellular Pol II. The CTD of unengaged Pol II is hypophosphorylated at Ser2 and Ser5. Pol II initiates at the transcription start site, and is Ser5-phosphorylated early in transcription. Later in transcription elongation, Ser2 is phosphorylated and Ser5 is gradually dephosphorylated. Influenza vRNPs in the nucleus target the Ser5-phosphorylated Pol II CTD, and bind to the m7G cap of nascent RNA on Pol II. RdRP in the vRNP cap snatches the nascent RNA, which is then used to prime viral transcription by influenza RdRP. The m7G capped, polyadenylated viral mRNA is exported to the cytoplasm through host pathways, and translated by host machinery.