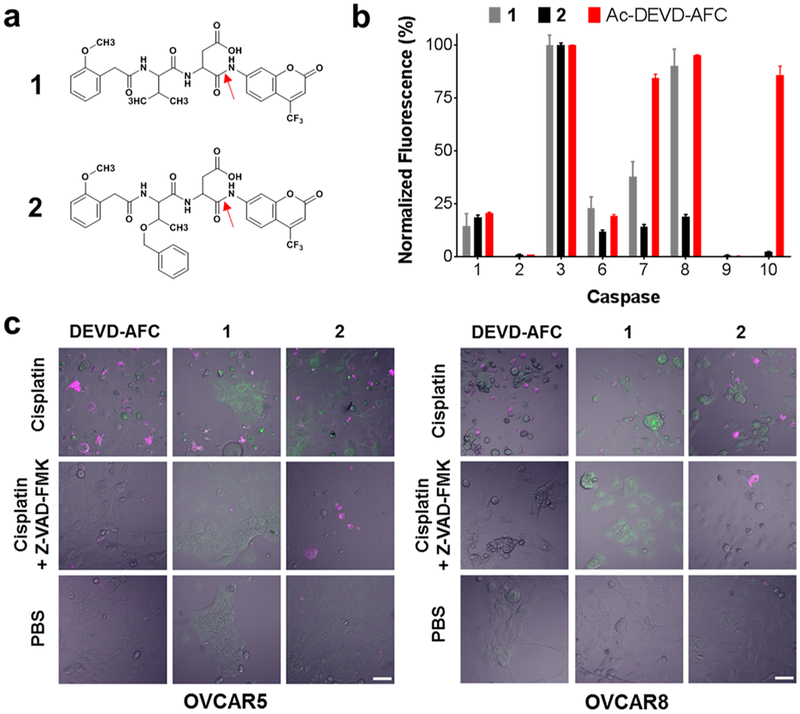
Figure 1.
Selectivity and intracellular activity of fluorescent caspase-3 substrates 1 and 2. (a) Structures of 1 and 2. The red arrows indicate the peptide bond cleaved by caspase-3. (b) Caspase specificity of 1, 2, and Ac-DEVD-AFC. Substrate concentrations equal to 3 × Km were cleaved in vitro by 1 U each of a panel of caspase enzymes for 2 h. Data for each probe was normalized to the caspase-3 activity for that probe. Bars represent mean ± standard deviation, n = 3. Substrate 2 showed the highest selectivity for caspase-3. (c) OVCAR-5 (left panel) and OVCAR-8 ovarian cancer cells (right panel) were pretreated for 48 h with 20 μM cisplatin, 20 μM cisplatin and 20 μM Z-VAD-FMK, or PBS. Then, cells were treated with 1 μ/mL Annexin-V-AF568 (magenta) and 100 μM of Ac-DEVD-AFC, 1, or 2 (green) for 2 h at 37 °C followed by confocal microscopy. In contrast to 1, uptake and cleavage of compound 2 was sharply inhibited following caspase-3 inactivation by Z-VAD-FMK. Scale bar 50 μm.