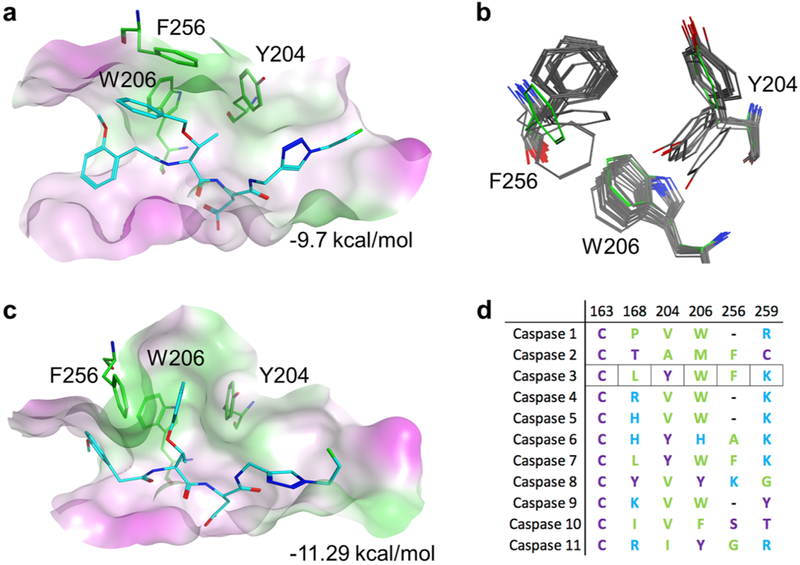
Figure 3.
Active site modeling of caspase-3 with substrates. (a) Active site of caspase-3 with pharmacophore docked pose of 16 in the closed configuration. Green represents hydrophobic and magenta hydrophilic surfaces. (b) Flexibility of the caspase-3 S2 pocket observed in crystal structures compared to the modeled closed conformation (green residues). (c) Binding of compound 16 in a flexible model of the caspase-3 active site. (d) Sequence alignment of the residues that define the S2 pocket of human caspases.