Abstract
Plasmablastic lymphoma (PbL) is a rare and aggressive B-cell malignancy with large neoplastic cells, most of them resembling plasmablasts that have a CD20-negative phenotype. Although initially described as being associated with HIV, over the years it has also been identified in patients with solid organ transplant and immunocompetent patients. Little is known about molecular basis that drives PbL, and still its diagnosis remains challenging given its rarity. However, proper recognition of its clinical characteristics, localization, and morphological features can establish a correct diagnosis of PbL within the spectrum of CD20-negative large B-cell lymphomas (LBCLs). PbL is characterized by CD20 and PAX5 negativity together with the expression of CD38, CD138, MUM1/IRF4, Blimp1, and XBP1 plasmacytic differentiation markers. It is usually associated with Epstein–Barr virus infections, and MYC gene rearrangements. PbL should be carefully differentiated from other CD20-negative B-cell neoplasms, ie, primary effusion lymphoma, anaplastic lymphoma kinase-positive (ALK) large B-cell lymphoma, and LBCL in human herpesvirus 8-associated multicentric Castleman disease. Despite our improved understanding of this disease, its prognosis remains dismal with short overall survival. There is no standard of care for this entity. Several chemotherapy combinations have been used with hardly any differences on its outcome. Thus, new approaches with the addition of novel molecules are needed to overcome its poor prognosis. Our current understanding and knowledge of PbL relies primarily on case reports and small case series. In this review, we revise through an extensive literature search, the clinical and biological characteristics of this entity, and the potential therapeutic options.
Keywords: plasmablastic lymphoma, review
Introduction
Plasmablastic lymphoma (PbL) is an aggressive B-cell malignancy highly associated with HIV.1 A fraction of large B-cell lymphomas (LBCL) share a plasmablastic differentiation with an aggressive behavior, refractoriness to chemotherapy, and poor prognosis in most cases.2 They are characterized by a gradual expression of transcription factors associated with the plasmacytic differentiation, CD38, CD138, MUM1, Blimp1, and XBP1, with decreased expression of CD20 and PAX5.3 In addition to PbL, plasmablastic/plasmacytic differentiation is well-documented in other entities such as primary effusion lymphoma, LBCL in human herpesvirus 8 (HHV-8)-associated multicentric Castleman disease, and anaplastic lymphoma kinase-positive LBCL. These constitute a subgroup of lymphomas with heterogeneous clinical, histological, and genetic features posing diagnostic challenges to pathologists.4 PbL is the most common and well-described subtype. Nevertheless, this entity is extremely rare, and its clinicopathological features and optimal treatment strategies remain obscure.5
Pathogenesis and biological features
PbL was first described in 1997 by Delecluse et al6 in patients with HIV. It has later been reported in patients with solid organ transplantation, other immunosuppressed, and even immunocompetent patients.7
PbL cells show immunoblastic morphology and immune-phenotype with plasmacytic differentiation markers including positivity for CD38, CD138, MUM1, Blimp1, and XBP1, and MYC, with variable expression for CD45, CD79a, EMA, and CD30. Although it generally shows no expression of B-cell markers (CD20 and PAX5), CD20 can be weakly positive in about 10% of the cases.1,4 The cell of origin of the PbL is thought to be the plasmablast, an activated B lymphocyte that has gone through the process of somatic hypermutation and class-switching recombination.1
The biological bases of PbL are not yet completely known. Different data suggest that there is a close relationship with immunodeficiency conditions in a large proportion of the cases.6,8–10 In this sense, there is a clear association with HIV infection, but also with other immunosuppressed status, such as in the context of solid organ transplant patients. Yet, the diagnosis of PbL cases in immunocompetent patients is also well-known, and patients usually are classified into three groups based on their immune status: HIV-positive patients, posttransplant patients, and immunocompetent patients.7,11,12
However, when analyzing the third group of immunocompetent patients in detail, a nonnegligible percentage of cases show relatively immunosuppressed conditions. A recent analysis by the Lysa Group of HIV-negative and nontransplanted cases showed, in 55 of 62 patients, the presence of factors associated with a certain degree of immunosuppression, such as autoimmune diseases or chronic systemic inflammatory diseases, history of previous neoplasms, or an age over 60 years that could be related to immunosenescence.13 Interestingly, they reported four cases that developed a PbL on a local inflammation. Only 5% of the cases of the Lysa Group global series could not be associated with any state of immu-nosuppression.13 PbL can develop in HIV-positive patients without a severe immunosuppression state. More than 50% of the cases of PbL in patients with HIV, in this series, were diagnosed in patients who received combined antiretroviral therapy (cART) and had CD4 counts greater than 200 cells/µL.13 The prevalence of HIV-positive and HIV-negative cases is not well-defined and ranges from 63% in some case reviews to 44% in the case registry of the National Cancer Data Base, or 41% in the Lysa Group cohort.13,14
A very high percentage of PbL cases show an association with Epstein–Barr virus (EBV) infection. EBV-encoded small RNA expression has been described in 80% of the HIV-positive cases and ~50% of the HIV-negative cases. This suggests an important role of EBV in the pathogenesis of PbL.12–15 However, there is a proportion of EBV-negative cases in the different series reported, and neither HIV status nor EBV seem to confer different patterns of gene expression in PbL.16
Beyond the association of PbL with immunosuppression and EBV infection, genetic studies have revealed frequent complex karyotypes. Several groups have found genetic alterations comprising the MYC oncogene that cause overexpression of the MYC protein. The most frequent alterations are translocations of MYC with IG (in ~50% of cases), together with amplifications of MYC.17,18 Moreover, overexpression of MYC can also be detected by immunohistochemistry in a large proportion of cases, which suggests a central role of MYC in the pathogenesis of this lymphoma.11,19 Finally, recurrent mutations in PRDM1, a gene that encodes the Blimp1 protein, have recently been described in 8 of 16 cases analyzed. These mutations in PRDM1 could alter the regulation of different targets, including MYC, in these lymphomas.20 In fact, loss of Blimp1 protein expression has recently been found to be associated with MYC overexpression, and decreased expression of p53 tumor-suppressor molecules in ABC-like diffuse large B-cell lymphoma (DLBCL).21
Clinical features, survival, and prognostic factors
PbL is more predominant in males (75% of the cases in the various analyzed series). At diagnosis, PbL commonly presents in extranodal regions, the most frequent being the oral cavity, the digestive tract, and the skin. Most patients present at an advanced stage (III or IV), with frequent bone marrow involvement and presence of B symptoms (40% of patients). However, there are differences in the clinical presentation of patients with different immunological status. Location of PbL in HIV-negative patients is more heterogeneous than in HIV-positive patients, and bone marrow involvement, as well as B symptoms are less frequent. Although lymph node involvement is less frequent at diagnosis, it has been described in up to 30% of posttransplant patients.6,8,9,11–13,22,23 PbL can be diagnosed as the final event (transformation) of an indolent lymphoproliferative syndrome.20 Cases after an acute lymphoblastic leukemia or DLBCL have also been described.20,24
The median age at diagnosis is around 50 years. However, the age of presentation is lower in HIV-positive patients, with a median age of 40 years, than in non-HIV patients, where the median age is above 50 years. This reinforces the concept that age-related senescence can play a relevant role in a proportion of HIV-negative cases.7,8,12–20,22–25
The prognosis of patients with PbL is generally poor with a median overall survival (OS) of 6–19 months, and no clear differences between HIV-positive and HIV-negative patients.7,8,12–20,22–25 A review of 300 patients with PbL showed a median survival of 10 months in HIV-positive patients, 11 months in HIV-negative patients, and only 7 months in posttransplant patients.7 More recently, the Lysa series, which comprises the largest cohort of PbL reported so far, has shown better survival outcomes with a median OS of 32 months, greater than that reported before.13 A multivariate analysis of this cohort showed that good prognosis was positively associated with low International Prognostic Index (IPI) and achieving a complete response (CR).13 Although HIV status does not seem to determine differences in the prognosis and survival of patients, some studies have suggested that HIV-positive patients may have a better outcome compared to HIV-negative patients.13,25 This better survival could be explained, in part, by the immunological restoration with cART treatments and by the lower age of HIV-positive patients compared with the HIV-negative cases, which could be associated with more intensive chemotherapy treatments. Supporting the later hypothesis, a study of 481 patients with PbL, from the National Cancer Data Base, showed no impact of HIV status on survival when the analysis was stratified by age.14
The prognostic impact of EBV status is not clear and varies depending on the series and subgroups of patients analyzed. Some studies have found no prognostic differences in HIV-positive patients, while other studies suggest that EBV-positive patients may have a better prognosis in HIV-negative patients.7,25,26
Beyond the HIV and EBV status, IPI is able to stratify patients into different prognostic groups, and is probably the most used prognostic index.15,26,27 More recently, rearrangements or gains of MYC has been associated with a worse outcome and shorter survival in patients with PbL compared with patients without MYC alterations.7,12,15,28
Treatment
The range of treatments delivered to PbL patients has been extensive, from local control with radiotherapy in patients with localized disease to a variety of chemotherapy combinations.29 Chemotherapy treatments have included CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP-like regimens, Hyper-CVAD-MA (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, and high-dose methotrexate and cytarabibe), CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine), COMB (cyclophosphamide, Oncovin, methyl-CCNU, and bleomycin), and infusional EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin).17,30,31 Spontaneous regressions have been observed in HIV-infected patients after initiation of cART or highly active antiretroviral therapy.32,33
Patients with localized disease have a better prognosis, and in some circumstances are not subsidiary of aggressive treatments. In these cases, disease control has been achieved with radiotherapy or combining doxorubicin-based chemotherapy with radiation therapy.29,34 However, the experience with radiotherapy alone is limited.
For patients with disseminated disease, polychemotherapy achieves more than 50% of complete remissions (CRs), but ~70% of patients die of progressive disease, with an event-free survival of 22 months, and an OS of 32 months, being significantly longer in patients achieving a CR.13 To date, there are no standards of care defining the optimal therapeutic approach. CHOP has been the most common regimen used in PbL; however, NCCN guidelines do not consider an adequate therapy, and recommend more intensive regimens such as Hyper-CVAD-MA, CODOX-M/IVAC, or EPOCH (infusional) therapy.35 About 38% of 5-year progression-free survival (PFS) and a 40% of 5-year OS in patients with PbL, 40% received Hyper-CVAD-MA regimen.36 Although infusional EPOCH regimen has not proven to achieve better results in PbL patients than other regimens, its use is supported by the good results achieved in HIV-associated Non-Hodgkin’s Lymphoma, and is becoming the treatment of choice in many institutions.37
One step further is the autologous stem cell transplantation (ASCT). No prospective randomized trials have been conducted in this setting due to the difficulty of carrying out this study in such a rare disease. A limited number of cases coming from short series have been reported in both HIV and immunocompetent patients with PbL, in relapsed or refractory (R/R) patients as well as in first response after frontline treatment. In patients with HIV, the Italian Cooperative Group on AIDS and Tumors (GICAT) reported five cases in R/R patients, with two deaths (at 4 and 6 months after ASCT) and three patients in CR after 21–79 months.27,38 Moffitt Cancer Center presented the experience of two non-HIV patients with PbL in partial response (PR) that underwent an ASCT. One of them achieved a CR, the other had a progressive disease, and both died at 12 and 6 months after ASCT.39 With regard to frontline ASCT in patients with PbL after a CR or PR, there is a need to consolidate the response in patients with a poor prognosis after R/R to the firstline therapy.13,15 An analysis of 5 patients with PbL who underwent an ASCT after CHOP regimen from a multicenter Phase II study of GIGAT group, with the addition of two more patients belonging to the same group, showed that four of them continue in CR after 13–83 months, and one died 4 months after this procedure.27,40 With a median follow-up of 19.5 months for the entire series, the 2-year PFS and OS were 73% and 76%, respectively.
The literature on allogeneic stem cell transplantation (allo-SCT) in PbL is considerably more limited compared with ASCT. In 2009, Hamadani and Devine41 reported one patient with PbL in CR2 who underwent a reduced-intensity allogeneic stem cell transplantation (RIC-allo-SCT) from a matched unrelated donor, and was alive 2 years after transplantation. To date, allo-SCT in PbL has not shown great efficacy.42
Treatment approaches beyond the standard chemotherapy
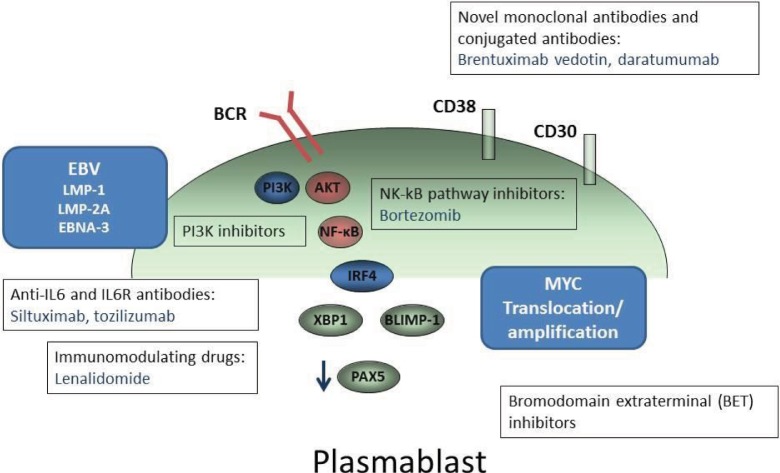
Given the low efficacy of the standard therapy, and the poor outcome of these patients, there is a need to move toward new therapeutic strategies by incorporating new agents. We here review some options split into two categories: 1) previously used drugs in small trials; and 2) potential future directions (Figure 1).
Figure 1.
New therapeutic targets in PbL.
Notes: Novel monoclonal antibodies and promising agents directed to inhibit different signaling pathways in PbL are represented.
Abbreviations: EBV, Epstein–Barr virus; PbL, plasmablastic lymphoma.
Drugs previously used in very short series
Bortezomib
Bortezomib is a proteasome inhibitor widely used for the treatment of multiple myeloma (MM). There are reports of its activity in lymphoma, mainly in non-Germinal center B-cell like DLBCL and mantle cell lymphoma.43,44 Bortezomib is the most reported new drug for PbL. It has been used as single agent, as well as in combination with chemotherapy. In a short series, out of six patients treated with bortezomib alone, five achieved a PR – two as frontline therapy and three as a salvage treatment.45 Bortezomib has been used in combination with CHOP as frontline therapy in three HIV-associated PbL patients, all of them achieved a CR, and two of them were alive 14 and 22 months after V-CHOP.46 However, the most frequently used chemotherapy regimen in combination with bortezomib has been infusional EPOCH. Castillo et al47 reported a series of three patients with complete resolution of disease measured by positron emission tomography scan and free of relapse at 12, 18, and 24 months. More recently, Dittus et al48 and Castillo et al49 have reported a series of 8 and 16 patients with a CR rate of 87.5% and 94%, respectively. Two patients in the latter series received an ASCT as consolidation. Dittus et al48 report a 2-year PFS and OS of 50%, and Castillo et al49 a 5-year OS of 63%. In relapsed patients, bortezomib has been used in combination with THP-COP (pirarubicin, cyclophosphamide, vincristine, and prednisone), ESHAP (etoposide, high-dose prednisolone, high-dose cytarabine, and platinum), ICE (Ifosfamide, carboplatin, and etoposide), bendamustine, rituximab, and DT-PACE with a CR rate of 16% and a PR rate of 84%.45
Lenalidomide
The immunomodulatory agent lenalidomide is widely used in treating patients with MM, and has also shown efficacy in non-GCB DLBCL.50 At present, there are two case reports showing a prompt but short response to single-agent lenalidomide in refractory PbL.51,52 Similarly, two cases were successfully treated with lenalidomide in combination with CHOP in one case, and with cyclophosphamide and dexamethasone in the other.53,54
Brentuximab vedotin (BrV)
CD30 expression is frequent in Hodgkin’s lymphoma and anaplastic large cell lymphoma, but it is infrequently seen in DLBCL. BrV is an antibody–drug conjugate of anti-CD30 that is well-established in the treatment of Hodgkin’s lymphoma and anaplastic T-cell lymphoma.55,56 There are some data describing a variable expression of CD30 in PbL, averaging ~30% of the cases.20 A dramatic response to BrV has been reported in a single case, but the patient died shortly due to previous disease disabilities.57
Anti-IL6 and IL6R antibodies (Siltuximab, Tozilizumab)
As mentioned before, EBV has been detected frequently in PbL.7 In cell lines derived from a patient with HIV-associated PbL harboring EBV (PBL-1), starvation of IL6 or addition of tozilizumab, an inhibitory antibody for the IL6 receptor, induced apoptosis in the PBL-1 cell line. This indicates an IL6 dependency of this cell line to proliferate and survive.58 Recently, a plasmablastic microlymphoma arising in HHV-8-associated multicentric Castleman disease in an HIV-negative patient that did not express EBV proteins nor CD30, showed a clinical response to siltuximab, an anti-IL6 antibody.59
Future directions
Potential therapeutic approaches for patients with PbL could include EBV-directed therapies such as antiviral agents and EBV-targeted cellular immunotherapy, not yet evaluated in these patients. Many antiviral agents do not act due to the quiescent nature of the EBV outside the lytic phase. Arginine butyrate upregulates the thymidine kinase and induces lytic phase. A Phase I/II trial of arginine butyrate in refractory patients with EBV-associated lymphomas, in combination with ganciclovir, showed limited activity except for post-transplantation lymphoproliferative disorders, in which a response rate of 83% was observed.60 EBV-specific cytotoxic T lymphocytes have several limitations, such as a long preparation time and the need for specialized facilities for their production.61 Some of these drawbacks could be overcome by using autologous EBV-specific CAR T-cells with CD30 as target (NCT01192464) or CAR T-cells directed to other specific target.
Immunostains for programmed cell death-ligand 1 (PDL-1) have showed a high or moderate expression in microenvironment cells of PbL (60%–72% of cells), and programmed death 1 (PD-1) in 22.5% of cases of PbL tumor cells.62 Both EBV-positive and EBV-negative PbL exhibited high expression of PD-1 and PDL-1. These findings constitute a strong rationale for testing anti-PD-1 or anti-PDL-1 monoclonal antibodies in this pathology.
MYC translocation has been found in more than 50% of patients with PbL, mainly in HIV-infected patients. More frequently, MYC appears overexpressed.7,20 The MYC gene has been considered untargetable because of a lack of binding domain.63 Recently, a small selective molecule, bromodomain extraterminal (BET) inhibitor (JQ1) has shown to induce cell cycle arrest by inhibition of MYC transcription.64 Thus, BET inhibitors could be tested in some of these patients.
The PI3K/Akt/m-TOR signaling pathway is another major pathway of IL-6 signaling, which occurs downstream of IL-6R/gp130/Jak. Inhibition of the PI3K/Akt/m-TOR pathway may induce cell death in a plasmablastic cell line (PBL-1), which suggests that inhibitors of this type could be potential therapeutic agents for PbL.58
Finally, daratumumab, an antibody directed against CD38, has shown an outstanding activity in patients with R/R MM.65,66 PbL exhibits a strong expression of CD38, and could be an optimal target for the treatment of this disease. Although several cases have been treated with daratumumab as salvage therapy, there are still no reports of its efficacy. Our personal experience is limited to a case with PbL treated at our institution with daratumumab alone in third line. The patient experienced a dramatic response shortly followed by a rapid progression of the disease (unpublished). We assume that results can be improved in combination with other drugs or chemotherapy. In fact, in a patient treated with daratumumab in combination with ICE followed by an ASCT as salvage therapy, reportedly relapsed after V-EPOCH, achieving a CR that persisted at the time of publication, 15 months later.49
Conclusion
PbL is a rare and aggressive subtype of LBCL. Although the WHO recognizes it as most commonly occurring with HIV infection, it extends beyond patients with HIV, with a significant proportion of cases occurring in post-transplant and immunocompetent patients. It is frequently associated with EBV, and in more than 50% of cases shows MYC trans-location or amplification. Awareness of this entity, as well as immunohistochemistry and correlation with clinical findings are crucial for establishing a correct diagnosis. Currently available chemotherapy fails to achieve good results, but prolonged remissions are possible. Clinical trials with novel immunotherapeutic agents and other drugs targeting some genes involved in the activation of NF-κB pathways, some of which are already ongoing, may show promising results.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Campo E, Stein H, Harris NL. Plasmablastic lymphoma. In: Swerdlow SH, Campo E, Harris NL, editors. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2017. pp. 321–322. [Google Scholar]
- 2.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17(12):3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Zhao S, Wang J, Chen J, Wen W, Zhang Q. CD20-negative diffuse large B cell lymphoma: a comprehensive analysis of 695 cases. Tumour Biol. 2016;37(3):3619–3637. doi: 10.1007/s13277-015-4205-5. [DOI] [PubMed] [Google Scholar]
- 4.Montes-Moreno S, Gonzalez-Medina AR, Rodriguez-Pinilla SM, et al. Aggressive large B-cell lymphoma with plasma cell differentiation: immunohistochemical characterization of plasmablastic lymphoma and diffuse large B-cell lymphoma with partial plasmablastic phenotype. Haematologica. 2010;95(8):1342–1349. doi: 10.3324/haematol.2009.016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montes-Moreno S, Montalbán C, Piris MA. Large B-cell lymphomas with plasmablastic differentiation: a biological and therapeutic challenge. Leuk Lymphoma. 2012;53(2):185–194. doi: 10.3109/10428194.2011.608447. [DOI] [PubMed] [Google Scholar]
- 6.Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89(4):1413–1420. [PubMed] [Google Scholar]
- 7.Morscio J, Dierickx D, Nijs J, et al. Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and post-transplant patients: single-center series of 25 cases and meta-analysis of 277 reported cases. Am J Surg Pathol. 2014;38(7):875–886. doi: 10.1097/PAS.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 8.Dong HY, Scadden DT, de Leval L, et al. Plasmablastic Lymphoma in HIV-Positive Patients. Am J Surg Pathol. 2005;29(12):1633–1641. doi: 10.1097/01.pas.0000173023.02724.1f. [DOI] [PubMed] [Google Scholar]
- 9.Song MK, Chung JS, Shin HJ, et al. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol. 2012;91(5):697–703. doi: 10.1007/s00277-011-1357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teruya-Feldstein J, Chiao E, Filippa DA, et al. CD20-negative large-cell lymphoma with plasmablastic features: a clinically heterogenous spectrum in both HIV-positive and -negative patients. Ann Oncol. 2004;15(11):1673–1679. doi: 10.1093/annonc/mdh399. [DOI] [PubMed] [Google Scholar]
- 11.Loghavi S, Alayed K, Aladily TN, et al. Stage, age, and EBV status impact outcomes of plasmablastic lymphoma patients: a clinicopathologic analysis of 61 patients. J Hematol Oncol. 2015;8(1):65. doi: 10.1186/s13045-015-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125(15):2323–2330. doi: 10.1182/blood-2014-10-567479. [DOI] [PubMed] [Google Scholar]
- 13.Tchernonog E, Faurie P, Coppo P, et al. Clinical characteristics and prognostic factors of plasmablastic lymphoma patients: analysis of 135 patients from the LYSA group. Ann Oncol. 2017;28(4):843–848. doi: 10.1093/annonc/mdw684. [DOI] [PubMed] [Google Scholar]
- 14.Qunaj L, Castillo JJ, Olszewski AJ. Survival of patients with CD20-negative variants of large B-cell lymphoma: an analysis of the National Cancer Data Base. Leuk Lymphoma. 2018;59(6):1–9. doi: 10.1080/10428194.2017.1387912. [DOI] [PubMed] [Google Scholar]
- 15.Castillo JJ, Furman M, Beltrán BE, et al. Human immunodeficiency virus-associated plasmablastic lymphoma: poor prognosis in the era of highly active antiretroviral therapy. Cancer. 2012;118(21):5270–5277. doi: 10.1002/cncr.27551. [DOI] [PubMed] [Google Scholar]
- 16.Chapman J, Gentles AJ, Sujoy V, et al. Gene expression analysis of plasmablastic lymphoma identifies downregulation of B-cell receptor signaling and additional unique transcriptional programs. Leukemia. 2015;29(11):2270–2273. doi: 10.1038/leu.2015.109. [DOI] [PubMed] [Google Scholar]
- 17.Valera A, Balagué O, Colomo L. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas. Am J Surg Pathol. 2011;34(11):1686–1694. doi: 10.1097/PAS.0b013e3181f3e29f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogusz AM, Seegmiller AC, Garcia R, Shang P, Ashfaq R, Chen W. Plasmablastic lymphomas with MYC/IgH rearrangement: report of three cases and review of the literature. Am J Clin Pathol. 2009;132(4):597–605. doi: 10.1309/AJCPFUR1BK0UODTS. [DOI] [PubMed] [Google Scholar]
- 19.Valera A, Colomo L, Martínez A, et al. ALK-positive large B-cell lymphomas express a terminal B-cell differentiation program and activated STAT3 but lack MYC rearrangements. Mod Pathol. 2013;26(10):1329–1337. doi: 10.1038/modpathol.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montes-Moreno S, Martinez-Magunacelaya N, Zecchini-Barrese T, et al. Plasmablastic lymphoma phenotype is determined by genetic alterations in MYC and PRDM1. Mod Pathol. 2017;30(1):85–94. doi: 10.1038/modpathol.2016.162. [DOI] [PubMed] [Google Scholar]
- 21.Xia Y, Xu-Monette ZY, Tzankov A, et al. Loss of PRDM1/BLIMP-1 function contributes to poor prognosis of activated B-cell-like diffuse large B-cell lymphoma. Leukemia. 2017;31(3):625–636. doi: 10.1038/leu.2016.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colomo L, Loong F, Rives S, et al. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol. 2004;28(6):736–747. doi: 10.1097/01.pas.0000126781.87158.e3. [DOI] [PubMed] [Google Scholar]
- 23.Harmon CM, Smith LB. Plasmablastic lymphoma a review of clinicopathologic features and differential diagnosis. Arch Pathol Lab Med. 2016;140(10):1074–1078. doi: 10.5858/arpa.2016-0232-RA. [DOI] [PubMed] [Google Scholar]
- 24.Marini C, Baldaia H, Trigo F, Castillo JJ. Transformation of a previously diagnosed diffuse large B-cell lymphoma to plasmablastic lymphoma. Am J Hematol. 2016;91(8):E324. doi: 10.1002/ajh.24375. [DOI] [PubMed] [Google Scholar]
- 25.Castillo JJ, Winer ES, Stachurski D, et al. Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Leuk Lymphoma. 2010;51(11):2047–2053. doi: 10.3109/10428194.2010.516040. [DOI] [PubMed] [Google Scholar]
- 26.Schommers P, Wyen C, Hentrich M, et al. Poor outcome of HIV-infected patients with plasmablastic lymphoma: results from the German AIDS-related lymphoma cohort study. AIDS. 2013;27(5):842–845. doi: 10.1097/QAD.0b013e32835e069d. [DOI] [PubMed] [Google Scholar]
- 27.Cattaneo C, Re A, Ungari M, et al. Plasmablastic lymphoma among human immunodeficiency virus-positive patients: results of a single center’s experience. Leuk Lymphoma. 2015;56(1):267–269. doi: 10.3109/10428194.2014.911867. [DOI] [PubMed] [Google Scholar]
- 28.Schommers P, Wyen C, Hentrich M, et al. Poor outcome of HIV-infected patients with plasmablastic lymphoma: results from the German AIDS-related lymphoma cohort study. AIDS. 2013;27(5):842–845. doi: 10.1097/QAD.0b013e32835e069d. [DOI] [PubMed] [Google Scholar]
- 29.Phipps C, Yeoh KW, Lee YS, et al. Durable remission is achievable with localized treatment and reduction of immunosuppression in limited stage EBV-related plasmablastic lymphoma. Ann Hematol. 2017;96(11):1959–1960. doi: 10.1007/s00277-017-3109-4. [DOI] [PubMed] [Google Scholar]
- 30.Zelenetz AD, Abramson JS, Advani RH, et al. Non-Hodgkin’s Lymphomas. J Natl Compr Canc Netw. 2010;8(3):288–334. doi: 10.6004/jnccn.2010.0021. [DOI] [PubMed] [Google Scholar]
- 31.Koizumi Y, Uehira T, Ota Y, et al. Clinical and pathological aspects of human immunodeficiency virus-associated plasmablastic lymphoma: analysis of 24 cases. Int J Hematol. 2016;104(6):669–681. doi: 10.1007/s12185-016-2082-3. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong R, Bradrick J, Liu YC. Spontaneous regression of an HIV-associated plasmablastic lymphoma in the oral cavity: a case report. J Oral Maxillofac Surg. 2007;65(7):1361–1364. doi: 10.1016/j.joms.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 33.Nasta SD, Carrum GM, Shahab I, Hanania NA, Udden MM. Regression of a plasmablastic lymphoma in a patient with HIV on highly active antiretroviral therapy. Leuk Lymphoma. 2002;43(2):423–426. doi: 10.1080/10428190290006260. [DOI] [PubMed] [Google Scholar]
- 34.Pinnix CC, Shah JJ, Chuang H, et al. Doxorubicin-based chemotherapy and radiation therapy produces favorable outcomes in limited-stage plasmablastic lymphoma: a single-institution review. Clin Lymphoma Myeloma Leuk. 2016;16(3):122–128. doi: 10.1016/j.clml.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis C. B-cell lymphomas. NCCN Clin Pract Guidel Oncol. 2017;Version 1 [Google Scholar]
- 36.Patel K, Feng L, Oki Y, et al. Plasmablastic lymphoma: 28 patient single institution experience. Blood. 2013;122(21):4310 LP–4310. [Google Scholar]
- 37.Sparano JA, Lee JY, Kaplan LD, et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115(15):3008–3016. doi: 10.1182/blood-2009-08-231613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Re A, Cattaneo C, Michieli M, et al. High-dose therapy and autologous peripheral-blood stem-cell transplantation as salvage treatment for HIV-associated lymphoma in patients receiving highly active antiretroviral therapy. J Clin Oncol. 2003;21(23):4423–4428. doi: 10.1200/JCO.2003.06.039. [DOI] [PubMed] [Google Scholar]
- 39.Liu M, Liu B, Liu B, et al. Human immunodeficiency virus-negative plasmablastic lymphoma: a comprehensive analysis of 114 cases. Oncol Rep. 2015;33(4):1615–1620. doi: 10.3892/or.2015.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Re A, Michieli M, Casari S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation as salvage treatment for AIDS-related lymphoma: long-term results of the Italian Cooperative Group on AIDS and Tumors (GICAT) study with analysis of prognostic factors. Blood. 2009;114(7):1306–1313. doi: 10.1182/blood-2009-02-202762. [DOI] [PubMed] [Google Scholar]
- 41.Hamadani M, Devine SM. Reduced-intensity conditioning allogeneic stem cell transplantation in HIV patients with hematologic malignancies: yes, we can. Blood. 2009;114(12):2564–2566. doi: 10.1182/blood-2009-06-229666. [DOI] [PubMed] [Google Scholar]
- 42.Al-Malki MM, Castillo JJ, Sloan JM, Re A. Hematopoietic cell transplantation for plasmablastic lymphoma: a review. Biol Blood Marrow Transplant. 2014;20(12):1877–1884. doi: 10.1016/j.bbmt.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Leonard JP, Kolibaba K, Reeves JA, et al. Randomized phase 2 open-label study of R-CHOP ± bortezomib in patients (Pts) with untreated non-germinal center B-cell-like (Non-GCB) subtype diffuse large cell lymphoma (DLBCL): results from the Pyramid trial ( NCT00931918) Blood. 2015;126(23):811. [PubMed] [Google Scholar]
- 44.Robak T, Huang H, Jin J, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372(10):944–953. doi: 10.1056/NEJMoa1412096. [DOI] [PubMed] [Google Scholar]
- 45.Guerrero-Garcia TA, Mogollon RJ, Castillo JJ. Bortezomib in plasma-blastic lymphoma: a glimpse of hope for a hard-to-treat disease. Leuk Res. 2017 Sep;62:12–16. doi: 10.1016/j.leukres.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez-Alvarez R, Gonzalez-Rodriguez AP, Rubio-Castro A, et al. Bortezomib plus CHOP for the treatment of HIV-associated plasmablas-tic lymphoma: clinical experience in three patients. Leuk Lymphoma. 2016;57(2):463–466. doi: 10.3109/10428194.2015.1050666. [DOI] [PubMed] [Google Scholar]
- 47.Castillo JJ, Reagan JL, Sikov WM, Winer ES. Bortezomib in combination with infusional dose-adjusted EPOCH for the treatment of plasmablastic lymphoma. Br J Haematol. 2015;169(3):352–355. doi: 10.1111/bjh.13300. [DOI] [PubMed] [Google Scholar]
- 48.Dittus C, Grover N, Ellsworth S, Tan X, Park SI. Bortezomib in combination with dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) induces long-term survival in patients with plasmablastic lymphoma: a retrospective analysis. Leuk Lymphoma. 2018;59(9):2121–2127. doi: 10.1080/10428194.2017.1416365. [DOI] [PubMed] [Google Scholar]
- 49.Castillo JJ, Guerrero-Garcia T, Baldini F, et al. Bortezomib plus EPOCH is effective as frontline treatment in patients with plasmablastic lymphoma. Br J Haematol. 2018:1–3. doi: 10.1111/bjh.15156. [DOI] [PubMed] [Google Scholar]
- 50.Fang C, Zhu D, Dong H, et al. Lenalidomide alone or in combination with chemotherapy treatment for subtypes of diffuse large B cell lymphoma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(7):10705–10713. [PMC free article] [PubMed] [Google Scholar]
- 51.Carras S, Regny C, Peoc’h M, et al. Dramatic efficacy of low dose lenalidomide as single agent in a patient with refractory gastric nonhuman immunodeficiency virus-associated plasmablastic lymphoma. Leuk Lymphoma. 2015;56(10):2986–2988. doi: 10.3109/10428194.2015.1016931. [DOI] [PubMed] [Google Scholar]
- 52.Bibas M, Grisetti S, Alba L. Patient with HIV-associated plasmablastic lymphoma responding to bortezomib alone and in combination with dexamethasone, gemcitabine, oxaliplatin, cytarabine, and pegfilgrastim chemotherapy and lenalidomide alone. J Clin Oncol. 2012;30(31):2012–2014. doi: 10.1200/JCO.2010.30.0038. [DOI] [PubMed] [Google Scholar]
- 53.Yanamandra U, Sahu KK, Jain N, Prakash G, Saikia U, Malhotra P. Plasmablastic lymphoma: successful management with CHOP and lenalidomide in resource constraint settings. Ann Hematol. 2016;95(10):1715–1717. doi: 10.1007/s00277-016-2732-9. [DOI] [PubMed] [Google Scholar]
- 54.Schmit JM, Delaune J, Norkin M, Grosbach A. A case of plasmablastic lymphoma achieving complete response and durable remission after lenalidomide-based therapy. Oncol Res Treat. 2017;40:1–2. 46–48. doi: 10.1159/000455146. [DOI] [PubMed] [Google Scholar]
- 55.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 56.Forero-Torres A, Fanale M, Advani R, et al. Brentuximab vedotin in transplant-naive patients with relapsed or refractory Hodgkin lymphoma: analysis of two phase I studies. Oncologist. 2012;17(8):1073–1080. doi: 10.1634/theoncologist.2012-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pretscher D, Kalisch A, Wilhelm M, Birkmann J. Refractory plasmablastic lymphoma-a review of treatment options beyond standard therapy. Ann Hematol. 2017;96(6):967–970. doi: 10.1007/s00277-016-2904-7. [DOI] [PubMed] [Google Scholar]
- 58.Mine S, Hishima T, Suganuma A, et al. Interleukin-6-dependent growth in a newly established plasmablastic lymphoma cell line and its therapeutic targets. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-10684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koenig G, Stevens TM, Peker D. Plasmablastic microlymphoma arising in human herpesvirus-8-associated multicentric Castleman disease in a human immunodeficiency virus-seronegative patient with clinical response to anti-interleukin-6 therapy. Histopathology. 2015;67(6):930–932. doi: 10.1111/his.12718. [DOI] [PubMed] [Google Scholar]
- 60.Perrine SP, Hermine O, Small T, et al. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood. 2007;109(6):2571–2578. doi: 10.1182/blood-2006-01-024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castillo JJ, Reagan JL, Bishop KD, Apor E. Viral lymphomagenesis: from pathophysiology to the rationale for novel therapies. Br J Haematol. 2014;165(3):300–315. doi: 10.1111/bjh.12788. [DOI] [PubMed] [Google Scholar]
- 62.Laurent C, Fabiani B, do C, et al. Immune-checkpoint expression in Epstein-Barr virus positive and negative plasmablastic lymphoma: a clinical and pathological study in 82 patients. Haematologica. 2016;101(8):976–984. doi: 10.3324/haematol.2016.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Darnell JE. Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2(10):740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 64.Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 66.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]