Abstract
Individuals with differences/disorders of sex development (DSD) have increased rates of infertility. For children and youth undergoing prophylactic gonadectomy for malignancy risk, our institution offers gonadal tissue cryopreservation, an experimental technique to preserve fertility cryopotential. An 11-year-old girl with partial androgen insensitivity syndrome presented for evaluation for fertility preservation in the setting of a planned bilateral gonadectomy at an outside institution. At presentation, the patient had begun puberty with an elevated serum androgen level and was experiencing undesired virilization. She expressed a strong female gender identity, an understanding of the various treatment options, and a preference for gonadectomy to prevent further virilization. After thorough counseling with the patient and family in our institution’s multidisciplinary DSD clinic, she underwent bilateral gonadectomy with gonadal tissue cryopreservation. Her gonadal pathologic examination demonstrated well-developed peripubertal testes, with present, albeit decreased, numbers of spermatogonial germ cells, decreased Leydig cells, and nonspecific degenerative changes. The patient and her family chose to maintain the cryopreserved tissue for the patient’s potential future use. To the best of our knowledge, the present case is the first reported case of gonadal tissue cryopreservation in a patient with partial androgen insensitivity syndrome. Storage of gonadal tissue is a feasible method of germ cell preservation in patients with DSD undergoing gonadectomy, although further research advances are required to facilitate development of this tissue into mature gametes capable of biological fertility.
Keywords: disorders of sex development, fertility preservation, spermatogonia, androgen-insensitivity syndrome, decision making
Many individuals with disorders/differences of sex development (DSD) are at risk of infertility secondary to anatomic barriers, abnormal gonadal development, abnormal hormone production or action, and prophylactic gonadectomy for malignancy risk. Although infertility has previously been assumed, recent data have suggested the fertility potential might be greater than previously thought for individuals with DSD, especially with innovative fertility preservation (FP) techniques [1]. The Gender & Sex Development Program and Program in Fertility Preservation and Hormone Restoration at Lurie Children’s Hospital work to offer FP to children and youth with DSD [2]. We report the case of a patient with partial androgen insensitivity syndrome (PAIS) who underwent cryopreservation of germ cell-containing gonadal tissue at the time of clinically indicated gonadectomy as a part of an institutional review board-approved FP protocol.
1. Case Report
An 11-year-old 46,XY female-identified patient with PAIS was referred to our institution for evaluation of FP in the setting of bilateral gonadectomy. The patient had been born full-term. Prenatal ultrasound examination findings had suggested the patient was female, and no genital atypia was noted at birth. However, at 6 months of age, she was noted to have clitoromegaly, prompting urologic consultation. The physical examination findings revealed bilateral enlarged labia, a common urogenital sinus, moderate clitoromegaly, and a palpable right gonad. A pelvic ultrasound examination demonstrated bilateral inguinal testes with no obvious uterus or vagina. Endocrine testing was consistent with a diagnosis of PAIS, and androgen receptor gene testing showed a mutation 2513A>G, previously unreported, but typical of those seen in PAIS.
The patient was first evaluated for gonadectomy for gonadal malignancy risk at 16 months of age. At that point, the family inquired about FP. However, little information about prepubertal FP was available at that time. The family elected not to pursue gonadectomy after the initial discussion. She underwent regular tumor surveillance throughout childhood, including physical examinations, measurement of α-fetoprotein and human chorionic gonadotropin, and testicular ultrasound examinations. All the results were reassuring.
At 11 years of age, clitoral enlargement was noted by an outside pediatric urologist, and laboratory testing demonstrated increasing levels of LH, FSH, and free and total testosterone, consistent with the onset of puberty (Table 1). The patient had consistently expressed an unequivocal female gender identity and stated that she did not want to “be a boy”; she, thus, wished to avoid the masculinizing effects of pubertal testosterone secretion. Therefore, bilateral gonadectomy was planned at an outside institution. The patient’s outside providers had originally planned to suppress her puberty with leuprolide acetate to allow for additional time for family discussion before surgery. However, insurance delays had made this approach untenable. Spironolactone was, thus, initiated in the interim.
Table 1.
Serum Endocrine Test Results From Age 6 mo to 11 y, 9 mo
| Hormone | Age | Prepubertal Normal Range | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 mo | 5 y, 2 mo | 8 y, 2 mo | 9 y, 1 mo | 10 y, 1 mo | 11 y, 2 mo | 11 y, 4 mo | 11 y, 7 mo | 11 y, 9 mo | Female | Male | |
| LH, IU/L | 2.3 | 0.2 | <0.2 | <0.2 | <0.1 | 1.4 | ND | ND | ND | <0.3 | <0.3 |
| FSH, IU/L | 1.0 | 1.4 | 1.7 | 2.2 | 1.8 | 2.5 | ND | ND | ND | 0.3–4 | 0.2–2.3 |
| Estradiol, pg/mL | ND | ND | 7 | 1.6 | ND | <2 | ND | ND | ND | ≤16 | NA |
| Testosterone, ng/dL | 16 | <20 | <20 | <12 | <12 | 84 | 134 | 126 | <12 | <10 | <150 |
| DHT, pg/mL | 136 | 11.9 | 14.4 | 13 | 24 | ND | ND | ND | ND | NA | <51 |
| SHBG, nmol/L | ND | ND | ND | 117 | 99 | 113 | 78 | ND | 85.3 | 27–149 | 27–149 |
| AMH, ng/mL | 305 | 151 | 108 | ND | 122 | 142 | ND | ND | ND | 0.3–6.3 | 20–190 |
| Inhibin, pg/mL | ND | 175 | 110 | 137 | 131 | 145 | ND | ND | ND | <130 | 35–170 |
| β-hCG, mIU/mL | ND | <1 | <1 | <1 | <1 | <1 | ND | ND | ND | <5 | <5 |
| AFP, ng/mL | ND | 1.7 | 1.9 | 2 | 2.4 | 2.5 | ND | ND | ND | <9 | <9 |
Patient’s hormone parameters over time, before and after gonadectomy at age 11 y, 6 mo.
Abbreviations: AFP, α-fetoprotein; AMH, anti-Müllerian hormone; β-hCG, human chorionic gonadotropin, β-subunit; NA, not applicable; ND, not determined.
Before surgery, the family again sought information about FP and was referred to our institution’s pediatric FP program. During counseling in our multidisciplinary DSD clinic (representative subspecialties include endocrinology, urology, psychology, pediatric surgery, social work, and genetic counseling), the patient again asserted a female gender identity and not only emphasized a strong desire to not undergo pubertal masculinization but also expressed distress at the possibility of further virilization. Because the patient’s parents had been open with her about her medical condition from a young age, she possessed a good understanding of her anatomy and knowledge of why her body had begun virilization at puberty (she described her gonads as “balls” and providing testosterone). She demonstrated a comprehensive knowledge of the treatment options and expressed preference for gonadectomy to prevent further virilization. During individual counseling with the patient, she recalled first being told she would not be able to have children around the age of 5 years and “not caring at all.” However, she acknowledged that her feelings had changed and expressed that she might want biological children in the future. The patient and her family were counseled both together and separately regarding the inability to predict her fertility potential, the experimental nature of gonadal tissue cryopreservation, the cost, and the potential uses for the tissue. Additionally, it was explained that any germ cells found would be spermatogonia and not concordant with her female gender identity and that use of the tissue would require either a female partner or a donor egg and gestational carrier. After counseling, the patient and her family chose to proceed with gonadectomy at our institution with gonadal tissue cryopreservation performed at surgery.
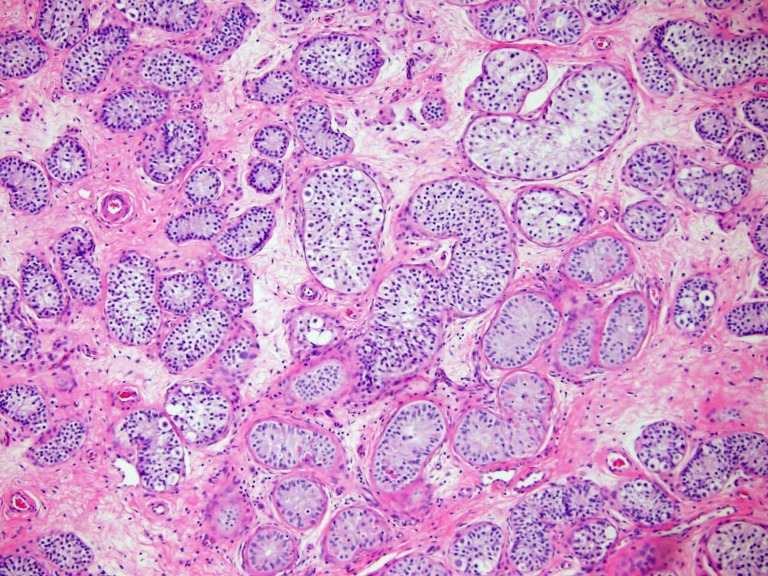
At 11 years and 6 months of age, the patient underwent bilateral inguinal gonadectomy with gonadal tissue cryopreservation under an institutional review board-approved protocol. Intraoperatively, surgeons noted that she had typically developed peripubertal testes in the distal inguinal canal and clitoromegaly with urogenital sinus and a blind-ending vaginal pouch. The gonads were bivalved intraoperatively, with one half submitted for pathological examination and the remaining tissue cryopreserved for the patient’s use. Pathologic examination revealed both gonads to be well-developed peripubertal testes with spermatogonial stem cells present (Fig. 1) and decreased numbers of Leydig cells, nonspecific degenerative changes, and no evidence of germ cell neoplasia. After further counseling with the family regarding the pathologic examination results, they elected to maintain the cryopreserved tissue for the patient’s potential future use.
Figure 1.
Gonadal pathology. Pathology of the patient’s gonadectomy specimen demonstrating the presence of spermatogonia, with a decreased number of germ cells and Leydig cells and nonspecific degenerative changes.
After gonadectomy, the patient started estrogen replacement therapy. Her testosterone level at 3 months after gonadectomy had decreased to prepubertal levels. At the 1-year follow-up examination, the patient had undergone appropriate interim female pubertal development and reported satisfaction with her appearance.
2. Discussion
To the best of our knowledge, the present study is the first time gonadal tissue cryopreservation of a patient with DSD undergoing gonadectomy has been reported. Fertility is an important concern for families of children with DSD, and many parents have expressed a desire for their child to maintain reproductive autonomy [3]. Although subfertility can be expected in patients with PAIS, both spontaneous fertility and fertility achieved through assisted reproductive techniques (ARTs) have been reported for adult men with PAIS [4]. Additionally, recent data have demonstrated the presence of germ cells in patients with complete androgen insensitivity syndrome, suggesting that they might have greater fertility potential than previously thought [1]. However, Cools et al. [5] also demonstrated an elevated risk of gonadal malignant transformation in patients with PAIS with nonscrotal gonads compared with those with complete androgen insensitivity (CAIS; 15% vs 0.8%), and gonadectomy has traditionally been recommended for patients with PAIS. Gonadectomy can also be chosen for some individuals with DSD when virilization is undesired by the patient, such as in our present patient.
Recent studies have advocated for consideration of fertility preservation (FP) and ART as options for patients with DSD [6, 7], although the optimal approach and timing for FP in these patients remains unclear. Cryopreservation of gonadal tissue as a method of preserving fertility potential has been best described in oncofertility studies and has been used in investigational protocols for prepubertal patients before the initiation of gonadotoxic therapies [8]. This technology, however, remains experimental. Ongoing research has been investigating several technologies that use cryopreserved testicular tissue to obtain mature spermatozoa, including transplantation of tissue, testicular tissue grafting, de novo testicular morphogenesis, testicular tissue culture, and in vitro spermatogenesis. Although no studies have reported successful spermatogenesis of preserved tissue in human populations, complete spermatogenesis yielding fertile offspring has been achieved in various animal models [9].
Many issues remain around gonadal tissue cryopreservation and should be discussed when counseling patients who wish to attempt FP. Prepubertal FP protocols cannot offer a guarantee of future fertility, and clinicians should be mindful that experimental treatments could lead to false hope for these patients and their families. For children with DSD, distress could be present regarding the potential discordance between the type of gonadal tissue and the patient’s gender identity. Additional ethical considerations include a pediatric patient’s ability to participate in the decision-making process, the potential for transmission of heritable genetic conditions to offspring, feelings of obligation to use frozen material, the burden placed on future partners if ART is required, and issues of cost and equitable distribution of resources [10]. Despite pediatric patients’ potentially limited ability to appreciate the complexities involved in FP, the tissue preserved is only available for use by patients once they have reached adulthood, at which time they could also choose to discard it.
3. Conclusion
Gonadal tissue cryopreservation represents a novel approach to FP in children with DSD. Storage of gonadal tissue is a feasible option for patients with DSD undergoing clinically indicated gonadectomy who might desire future biological parenthood. Further research advances are required to facilitate the development of this gonadal tissue into germ cells capable of biological fertility. Future studies will also be necessary to determine patient candidacy, the quality of germ cells at the time of FP, and the optimal timing for FP, as well as future fertility desires and the effect of procedure on children with DSD. Ethical and financial concerns should also be considered.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ART
assisted reproductive technique
- CAIS
complete androgen insensitivity syndrome
- DSD
differences/disorders of sex development
- PAIS
partial androgen insensitivity syndrome
References and Notes
- 1. Finlayson C, Fritsch MK, Johnson EK, Rosoklija I, Gosiengfiao Y, Yerkes E, Madonna MB, Woodruff TK, Cheng E. Presence of germ cells in disorders of sex development: implications for fertility potential and preservation. J Urol. 2017;197(3 Pt 2):937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finlayson C, Johnson EK, Chen D, Dabrowski E, Gosiengfiao Y, Campo-Engelstein L, Rosoklija I, Jacobson J, Shnorhavorian M, Pavone ME, Moravek MB, Bonifacio HJ, Simons L, Hudson J, Fechner PY, Gomez-Lobo V, Kadakia R, Shurba A, Rowell E, Woodruff TK. Proceedings of the Working Group Session on Fertility Preservation for Individuals with Gender and Sex Diversity. Transgend Health. 2016;1(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson EK, Rosoklija I, Shurba A, D’Oro A, Gordon EJ, Chen D, Finlayson C, Holl JL. Future fertility for individuals with differences of sex development: parent attitudes and perspectives about decision-making. J Pediatr Urol. 2017;13(4):402–413. [DOI] [PubMed] [Google Scholar]
- 4. Van Batavia JP, Kolon TF. Fertility in disorders of sex development: a review. J Pediatr Urol. 2016;12(6):418–425. [DOI] [PubMed] [Google Scholar]
- 5. Cools M, Looijenga LH, Wolffenbuttel KP, Drop SL. Disorders of sex development: update on the genetic background, terminology and risk for the development of germ cell tumors. World J Pediatr. 2009;5(2):93–102. [DOI] [PubMed] [Google Scholar]
- 6. Lee PA, Rogol A, Houk CP. Optimizing potential for fertility: fertility considerations for the pediatrician. Pediatr Clin North Am. 2011;58(5):1201–1215, x. [DOI] [PubMed] [Google Scholar]
- 7. Lee PA, Nordenström A, Houk CP, Ahmed SF, Auchus R, Baratz A, Baratz Dalke K, Liao LM, Lin-Su K, Looijenga LH III, Mazur T, Meyer-Bahlburg HF, Mouriquand P, Quigley CA, Sandberg DE, Vilain E, Witchel S; Global DSD Update Consortium. Global disorders of sex development update since 2006: perceptions, approach and care. Horm Res Paediatr. 2016;85(3):158–180. [DOI] [PubMed] [Google Scholar]
- 8. Johnson EK, Finlayson C, Rowell EE, Gosiengfiao Y, Pavone ME, Lockart B, Orwig KE, Brannigan RE, Woodruff TK. Fertility preservation for pediatric patients: current state and future possibilities. J Urol. 2017;198(1):186–194. [DOI] [PubMed] [Google Scholar]
- 9. Giudice MG, de Michele F, Poels J, Vermeulen M, Wyns C. Update on fertility restoration from prepubertal spermatogonial stem cells: how far are we from clinical practice? Stem Cell Res (Amst). 2017;21:171–177. [DOI] [PubMed] [Google Scholar]
- 10. Campo-Engelstein L, Chen D, Baratz AB, Johnson EK, Finlayson C. The ethics of fertility preservation for pediatric patients with differences (disorders) of sex development. J Endocr Soc. 2017;1(6):638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]