Abstract
Cardiac implantable electronic device–related infection is clinically challenging. Curative treatment commonly includes system removal. A case caused by Granulicatella adiacens occurred in a 32-year-old woman. Clinical course, literature review, and biofilm investigations enabled successful antibiotic management without system removal.
Keywords: biofilm infection, cardiac device-related infection, endocarditis, Granulicatella adiacens, nutritionally variant streptococci
Cardioverter defibrillator and cardiac resynchronization therapy devices are increasingly implanted. Infection related to these devices (ie, cardiac implantable electronic device [CIED]–related infection) is associated with significant morbidity and mortality and considerable healthcare costs [1]. Expert consensus statements and national guidelines have proposed treatment algorithms for CIED-related infections [2–5]. Curative treatment requires complete device system removal and antimicrobial therapy. Although lead extraction is a safe intervention in the majority of cases, complications are associated with high mortality rates.
There are considerable differences in biofilm production between various bacterial species and within each species of microorganism. These differences have the potential to be useful for decision making during the treatment of biofilm-related infection. We present a case of CIED-related infection and bioprosthesis endocarditis caused by Granulicatella adiacens. Results of antimicrobial susceptibility testing and biofilm investigations together with results from a literature review aided interdisciplinary decision making for the successful management of the infection without device system removal.
CASE REPORT
A 32-year-old woman was referred to our center with a diagnosis of CIED-related infection and bioprosthesis endocarditis. She was born with complex congenital heart disease (CHD) that included a complete transposition of the great arteries, a small ventricular septal defect and severe pulmonary valve stenosis. A Rashkind procedure was conducted in the neonatal period. When she was 6 years old, a Rastelli procedure was performed: left ventricular output was rerouted by a ventricular septal defect closure patch to the transposed aorta, and right ventricular output was rerouted by a surgically placed conduit to the pulmonary artery (RV-PA conduit; see Supplementary Figure 1). An epicardial pacemaker was implanted during the immediate postoperative course because of a complete atrioventricular block.
When the patient was 11 years old, her severely stenotic pulmonary conduit (a pulmonary homograft) was replaced with a new conduit containing a 25-mm biological valve prosthesis (Carpentier-Edwards). When she was 14 years old, an endovenous automatic implantable cardioverter defibrillator was implanted, after she was resuscitated because of ventricular tachycardia. A new right ventricular defibrillator electrode was implanted when she was 29 years old because of lead failure of the shock electrode.
In December 2015, the patient presented with a 2-day history of fever (38.8°C), chills, shortness of breath, and thoracic pain. A chest-radiograph showed no remarkable findings (see Supplementary Figure 2A). A computed tomographic (CT) scan demonstrated pulmonary embolism with subsequent infarction in the lower left lobe. These findings were subsequently confirmed by fludeoxyglucose F 18 positron emission tomography/CT (FDG PET/CT) (see Supplementary Figure 2B). G. adiacens grew in 3 sets of blood cultures (each consisting of an aerobic and an anaerobic bottle) obtained over 36 hours (0, 28, and 36 hours after admission). The first 2 pairs of blood cultures were obtained without antibiotic treatment. Empiric intravenous (treatment with amoxicillin-clavulanate was started 4 hours before the third set of cultures was obtained. Transthoracic and transoesophageal echocardiography was performed, along with FDG PET/CT, and none of these modalities revealed findings consistent with native valve, RV-PA conduit, or CIED infection.
Based on the presence of 1 major and 3 minor Duke criteria (major: continuous bacteremia with G. adiacens over 36 hours; minor: fever, predisposition, and septic pulmonary infarction), the diagnosis of CIED-related infection and conduit endocarditis was made. Targeted intravenous antimicrobial treatment consisted of penicillin G (4 million units every 4 hours) and gentamicin (3 mg/kg body weight [170 mg] once daily). Follow-up blood cultures remained negative. Removal of the implanted cardiac device system and replacement of the conduit were discussed. In light of her medical history and the surgical risks (third sternotomy), the patient was reluctant to undergo surgery for removal and replacement of the device.
Antimicrobial susceptibility investigations revealed that the causative pathogen displayed low minimal inhibitory concentrations (MICs). Biofilm assays indicated poor biofilm production. An interdisciplinary team decided to continue therapy with a conservative but curative treatment approach, together with therapeutic drug monitoring. After 2 weeks of penicillin-gentamicin combination therapy, treatment was switched to ceftriaxone (2 g once daily, given intravenously) for 4 weeks, followed by amoxicillin (1 g taken orally, 3 times daily) for another 6 weeks (ie, for a total treatment duration of 12 weeks). Several clinical, laboratory, and echocardiographic follow-up investigations over a 3-year period showed no relapse of infection.
METHODS AND RESULTS
Susceptibility Tests
MICs were determined using Etests (bioMérieux) on Mueller-Hinton sheep blood agar plates. Streptococcus pneumoniae American Type Culture Collection 49619 strain was used for quality control. The MIC results were as follows for penicillin, amoxicillin, ceftriaxone, rifampin, respectively: 0.006, 0.016, 0.016, and, 0.002 µg/mL.
Serum Concentrations of Administered Antimicrobial Agents
Antibiotic concentrations were measured via high-performance liquid chromatography-mass spectrometry. The penicillin serum concentration (trough level, 4 hours after last administration [infusion no. 21]) was 3.25 µg/mL at day 4 of treatment. The trough serum level of amoxicillin (oral intake no. 13) was 1.5 µg/mL at day 5 of amoxicillin therapy. The trough serum values for penicillin G and amoxicillin were more than 540 and 93 times, respectively, above the MICs. Considering protein binding proportions of 45%–65% for penicillin G and 15%–20% for amoxicillin, the results indicated that the free drug concentrations remained above the MIC (fT > MIC of 100%) for planktonic bacteria in serum.
Biofilm Formation Assays
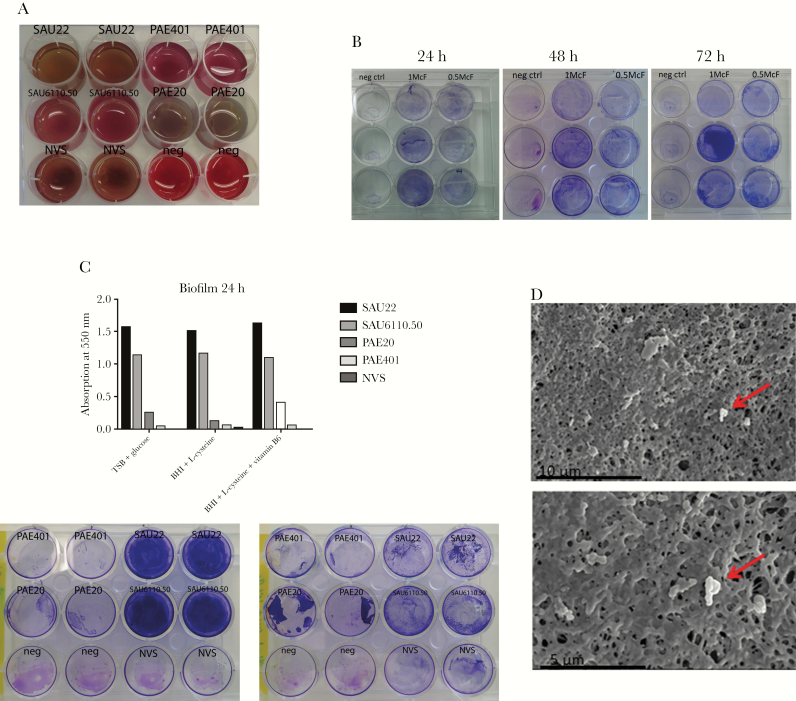
The extent of biofilm formation was quantified via absorption of crystal violet dye at 550 nm, after 24, 48 and 72 hours of incubation. (See Supplementary Material for details on methods.) Four bacterial strains were used for comparison: 2 Staphylococcus aureus strains (a clinical isolate obtained from a patient with a proved CIED-related infection [SAU6110.50] and a laboratory quality control strain [SAU22]) and 2 Pseudomonas aeruginosa strains (a clinical isolate from a patient with cystic fibrosis [PA401] and a laboratory quality control strain [PA20]).
Aggregation of bacteria was macroscopically evident in broth incubating P. aeruginosa (Figure 1A). The results of poor crystal violet staining of G. adiacens were seen, along with marked differences between S. aureus or P. aeruginosa and G. adiacens (Figure 1B and 1C).
Figure 1.
A, Broth media incubating 2 Staphylococcus aureus strains (clinical isolate obtained from a patient with a proved cardiac implantable electronic device [CIED] infection [SAU6110.50] and a laboratory quality control strain [SAU22]) and 2 Pseudomonas aeruginosa strains (clinical isolate from a patient with cystic fibrosis [PA401] and a laboratory quality control strain [PAE20]). Aggregation of bacteria was macroscopically evident in broth incubating P. aeruginosa (strain PAE20). B, Crystal violet (CV) staining with inocula of 0.5 and 1.0 McFarland (McF) Granulicatella adiacens and incubation durations of 24, 48, and 72 hours. Overall, the dye uptake is poor. C, Differences between the CV staining of S. aureus or P. aeruginosa and G. adiacens in the results from absorption measurements (top) and wells (bottom). D, Scanning electron microscopic images of the polymethylmethacrylate (PMMA) bead biofilm formation assay show few adherent bacteria (red arrows) detected on the PMMA surface. Abbreviations: BHI, brain-heart infusion broth; neg ctrl, negative control; neg, negative control; NVS, nutritionally variant streptococci; TSB, tryptic soy broth.
We then performed biofilm formation assays on bone cement beads and coverslips (see Supplementary Material for details). After 48-hour incubation and removal of nonsessile bacteria, the number of colony-forming units in biofilm was quantified via sonication and visualized with a scanning electron microscope. No bacteria were cultured after sonication (0 colony-forming units/mL). Consistent with these results, almost no adherent bacteria were found in investigations with a scanning electron microscope (Figure 1D).
Review of the Literature
The search strategy and details on included cases are listed in the Supplementary Material (Supplementary Table 1). We identified 15 cases with an infection related to an intravascular foreign body and due to either Granulicatella or Abiotrophia spp. The median patient age was 61.5 years (range, 18–79 years); 3 patients (20%) were female, and 12 (80%) were male. Infection was related to a vascular prosthesis in 2 patients, to a prosthetic valve in 11 (73.3%), and to both in 1. One infection was related to a pacemaker and a bioprosthesis, a condition similar to our case [6]. The aortic valve (8 cases [53.3%]) was most commonly involved. In 10 cases (66.6%), the causative microorganism was Granulicatella spp., and in 5 (33.3%) it was Abiotrophia spp. In 9 cases (60%), no surgical procedure was performed. Death occurred in 1 patient. The other 14 cases were classified as cured. A follow-up examination was available in 11 patients, and in 6 of them the follow-up was ≤6 months.
Discussion
The prevalence of CHD has increased in the past decades. This is true for children and even more so for adults [7]. In a 2018 nationwide study from Denmark, the live-birth incidence of CHD was 1.2% and constant over the past 20 years [8]. In Switzerland, every year, approximately 700 children are born with CHD. Half of them have moderately complex (eg, coarctation of the aorta) or complex (eg, transposition of the great arteries) defects. More than 90% of children with repaired CHD survive into adulthood [9]. Consequently, the number of adult patients with CHD has outgrown the pediatric CHD cohort. Two of 3 patients with CHD are adults [7].
There is an increased risk for infective endocarditis in adults with CHD. In the CONCOR adult CHD registry, the incidence of endocarditis was 1.33 cases per 1000 person-years. In this cohort, valve-containing prosthetics were independently associated with a greater risk for endocarditis, even 12 months beyond implantation (hazard ratio, 5.26; 95% confidence interval, 3.52–7.86) [10]. In our case, no vegetation was observed in repeated echocardiographic images, although typical endocarditis features in RV-PA valved conduits are difficult to detect with echocardiography [11]. The sensitivity of the FDG PET/CT scan for “lead infection” was reported as 24% (95% CI 5%–54%) for the standard method (1 hour) and 61% (95% CI 32%–86%) for the delayed method (3 hours) in 1 study [12]. A meta-analysis of this method was proved to have good overall accuracy for the diagnosis of CIED-related infections, with a pooled sensitivity of 87% and a specificity of 94% [13]. Although FDG PET/CT had negative findings in our case, prolonged bacteremia and septic pulmonary infarction were convincing arguments to postulate CIED-related infection and bioprosthesis endocarditis. We were unable to determine whether only part of the device was infected (eg, lead infection) or whether the infection involved both bioprosthesis and native valves. In addition, Erba et al [14] reported a low sensitivity for diagnosing device-related infections using Duke criteria.
Although conservative treatment for prosthetic valve endocarditis may be attempted, removal of the complete system, including electrodes, is currently regarded as the standard of care for CIED-related infection. The major complication rate ranges from 0.7% to 1.9%, depending on the extraction technique [15]. Major complications may have a high mortality risk, particularly in patients with multiple and older leads, internal cardioverter and defibrillator leads, or calcified leads and in adults with CHD. Complications include hemothorax, pericardial effusion, tamponade, pneumothorax, valve damage, and thromboembolism. CIED system removal requires a team approach by an interventional rhythmologist, a cardiac surgeon, a cardiac technician, and an anesthesia team capable of providing echocardiographic guidance. Thus, removal is associated with significant costs, and there are arguments for conservative treatment.
Nutritionally variant streptococci have been regarded as difficult to eradicate in infective endocarditis. This belief is based mainly on treatment failures in 12 of 29 reviewed cases (41%), according to a report published in 1987 [16]. In that study, bacterial failure was defined as positive blood cultures after 7 days of antibiotic therapy, relapse after a course of therapy with appropriate antibiotics, or a positive valve culture after therapy. A 2018 study on 76 infective endocarditis cases [17], collected from 2000 to 2015, showed a low overall mortality rate (13.2% in cases due to Abiotrophia spp., 5.2% in those due to Granulicatella spp.). This observation is in line with the results from our literature review. Nutritionally variant streptococci may not always be difficult to treat, considering that 9 of 15 cases with an infection related to a foreign body were successfully treated conservatively. There are considerable differences in MICs, however, when comparing susceptibility testing results.
Recommendations for antimicrobial management of CIED infection are similar to those for infective endocarditis (ie, ≥4 to 6 weeks for complicated infection) [2–5]. There are, however, no uniform recommendations in the context of device retention. It is reasonable that intravenous treatment is followed by oral suppressive therapy. The total treatment duration may be decided on an individual case basis, considering multiple variables. In our patient, excellent treatment response, the good prognosis reflected in the literature review (Supplementary Table 1), and the lack of infection signs with repeated echocardiography and FDG PET/CT follow-up aided the decision to stop treatment after 3 months.
In CIED-related infection, identifying pathogens with poor adherence properties may be a step toward a treatment concept without device removal. In the past few years, several diagnostic concepts in the field of biofilm investigation have been developed and critically reviewed [18, 19]. The determination of minimal biofilm eradication concentration may reflect a phenotypic pattern that is difficult to interpret. In our investigations, biofilm production was assessed with 2 different assays that revealed similar results. We assessed biofilm production only in a static system of adherence to acrylic glass and bone cement and not adherence to wire material. There are no data demonstrating the relation of biofilm assay results to clinical outcomes in CIED-related infection. However, biofilm investigations of the causative pathogen together with other clinical variables may aid interdisciplinary decision making for successful antibiotic management without device system removal.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank our patient for supporting the publication of this article. We acknowledge many physicians, scientists, nurses, and other healthcare workers for their contribution to this case, including Alessandro Spirito, Valentin Gisler, Cedric Hirzel, Katja Tiefenthaler, Marco Rossi, Laurent Arthur Decosterd, and employees of the Institute for Infectious Diseases, University of Bern, Switzerland. Barbara Every of BioMedical Editor, St Albert, Alberta, Canada, provided English-language editing.
Financial support. This work was supported by the Velux Foundation (grant 724 to P. S.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sohail MR, Henrikson CA, Braid-Forbes MJ, et al. . Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med 2011; 171:1821–8. [DOI] [PubMed] [Google Scholar]
- 2. Wilkoff BL, Love CJ, Byrd CL, et al. ; Heart Rhythm Society; American Heart Association Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009; 6:1085–104. [DOI] [PubMed] [Google Scholar]
- 3. Baddour LM, Epstein AE, Erickson CC, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–77. [DOI] [PubMed] [Google Scholar]
- 4. Sandoe JA, Barlow G, Chambers JB, et al. . New guidelines for prevention and management of implantable cardiac electronic device-related infection. Lancet 2015; 385:2225–6. [DOI] [PubMed] [Google Scholar]
- 5. Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. . 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017; 14:e503–51. [DOI] [PubMed] [Google Scholar]
- 6. Jeng A, Chen J, Katsivas T. Prosthetic valve endocarditis from Granulicatella adiacens (nutritionally variant streptococci). J Infect 2005; 51:e125–9. [DOI] [PubMed] [Google Scholar]
- 7. Marelli AJ, Ionescu-Ittu R, Mackie AS, et al. . Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014; 130:749–56. [DOI] [PubMed] [Google Scholar]
- 8. Lytzen R, Vejlstrup N, Bjerre J, et al. . Live-born major congenital heart disease in Denmark: incidence, detection rate, and termination of pregnancy rate from 1996 to 2013. JAMA Cardiol 2018; 3:829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moons P, Bovijn L, Budts W, et al. . Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 2010; 122:2264–72. [DOI] [PubMed] [Google Scholar]
- 10. Kuijpers JM, Koolbergen DR, Groenink M, et al. . Incidence, risk factors, and predictors of infective endocarditis in adult congenital heart disease: focus on the use of prosthetic material. Eur Heart J 2017; 38:2048–56. [DOI] [PubMed] [Google Scholar]
- 11. Miranda WR, Connolly HM, Bonnichsen CR, et al. . Prosthetic pulmonary valve and pulmonary conduit endocarditis: clinical, microbiological and echocardiographic features in adults. Eur Heart J Cardiovasc Imaging 2016; 17:936–43. [DOI] [PubMed] [Google Scholar]
- 12. Leccisotti L, Perna F, Lago M, et al. . Cardiovascular implantable electronic device infection: delayed vs standard FDG PET-CT imaging. J Nucl Cardiol 2014; 21:622–32. [DOI] [PubMed] [Google Scholar]
- 13. Juneau D, Golfam M, Hazra S, et al. . Positron emission tomography and single-photon emission computed tomography imaging in the diagnosis of cardiac implantable electronic device infection: a systematic review and meta-analysis. Circ Cardiovasc Imaging 2017; 10(4). pii: e005772. [DOI] [PubMed] [Google Scholar]
- 14. Erba PA, Sollini M, Conti U, et al. . Radiolabeled WBC scintigraphy in the diagnostic workup of patients with suspected device-related infections. JACC Cardiovasc Imaging 2013; 6:1075–86. [DOI] [PubMed] [Google Scholar]
- 15. Wazni O, Wilkoff BL. Considerations for cardiac device lead extraction. Nat Rev Cardiol 2016; 13:221–9. [DOI] [PubMed] [Google Scholar]
- 16. Stein DS, Nelson KE. Endocarditis due to nutritionally deficient streptococci: therapeutic dilemma. Rev Infect Dis 1987; 9:908–16. [DOI] [PubMed] [Google Scholar]
- 17. Tellez A, Ambrosioni J, Llopis J, et al. . Epidemiology, clinical features, and outcome of infective endocarditis due to Abiotrophia species and Granulicatella species: report of 76 cases, 2000–2015. Clin Infect Dis 2018; 66: 104–11. [DOI] [PubMed] [Google Scholar]
- 18. Magana M, Sereti C, Ioannidis A, et al. . Options and limitations in clinical investigation of bacterial biofilms. Clin Microbiol Rev 2018; 31:e00084–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coenye T, Goeres D, Van Bambeke F, Bjarnsholt T. Should standardized susceptibility testing for microbial biofilms be introduced in clinical practice? Clin Microbiol Infect 2018; 24:570–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.