Figure 3.
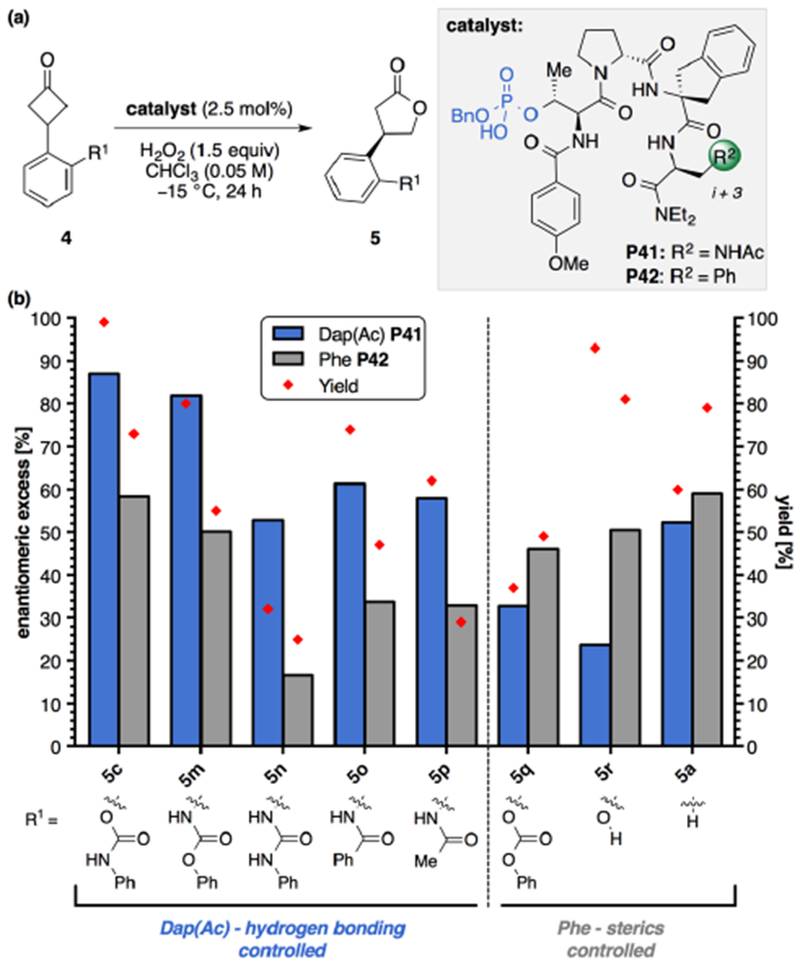
(a) General reaction scheme for BV oxidation with peptide catalyst and (b) structure–selectivity study with P41 and Phe analog (P42). Reaction conditions: Cyclobutanone 4 (0.05 mmol, 1.0 equiv), catalyst (2.5 mol%), and H2O2 (30% w/w aq. 1.5 equiv) at −15 °C with [4c] = 0.05 M CHCl3. Yield was determined by 1H NMR analysis of the crude reaction mixture using an internal standard (1,3,5-trimethoxybenzene). Enantiomeric ratios were by CSP-HPLC analysis. Reported results are the average of two trials. The absolute configuration of products 5m–r were assigned in analogy to 5e and 5f. The absolute configuration of 5a was assigned as (R)-(−)-5a by comparison of the measured optical rotation to literature values.17a
