Table 4.
Carbamate substrate scope.a
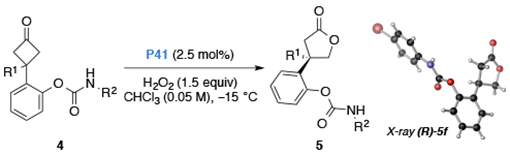 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | time (h) | yield (%)b | erc |
| 1 | H | C6H5 (c) | 24 | 99 | 94:6 |
| 2 | H | 4-OMeC6H4 (d) | 24 | 99 | 93:7 |
| 3 | H | 4-CF3C6H4 (e) | 24 | 98 | 93:7 |
| 4 | H | 4-BrC6H4 (f) | 24 | 94 | 94:6 |
| 5 | H | 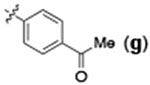 |
24 | 95 | 92:8 |
| 6 | H | 4-f-BuC6H4 (h) | 24 | 97 | 94:6 |
| 7 | H | 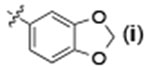 |
24 | 96 | 93:7 |
| 8 | H | CH3CH2 (j) | 84 | 96 | 85:15 |
| 9 | H | C6H5CH2 (k) | 72 | 95 | 82:18 |
| 10 | CH3 | C6H5 (l) | 72 | 94 | 80:20 |
Reaction conditions: Cyclobutanone 4 (0.10 mmol, 1.0 equiv), P41 (2.5 mol%), and H2O2 (30% w/w aq. 1.5 equiv) at −15 °C with [4c] = 0.05 M CHCl3. Reported results are the average of two trials.
Isolated yield after chromatography.
Enantiomeric ratios were determined by CSP-HPLC analysis.
See supporting information for details on assignment of absolute configuration. The configuration of products 5c-d, 5g–l were assigned in analogy to 5e and 5f.
