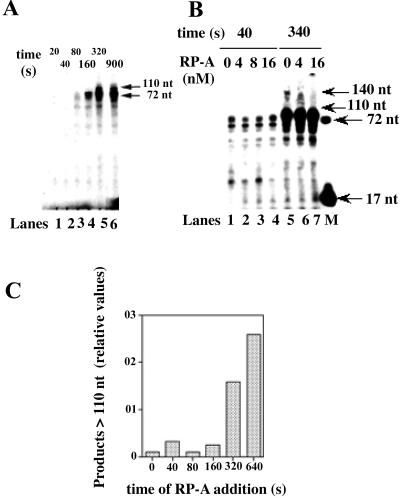
Figure 3.
RP-A limits the size of the DNA strand displaced by pol δ. (A) Pol δ (0.1 unit), was incubated in the presence of 12 nM (3′-OH ends) 72:17-mer template-primer and 16 nM RP-A (as heterotrimer), under the conditions described in Materials and Methods. Reactions were stopped at the indicated time points and the products were resolved on a 14% PAA/7 M Urea gel. Lane M: 5′-labeled linear 70-mer and 17-mer were loaded as markers. (B) Pol δ (0.1 unit) was incubated in the presence of 12 nM (3′-OH ends) 72:17-mer template-primer and increasing amounts of RP-A under the conditions described in Materials and Methods. Parallel reactions were stopped after 40 s (lanes 1–4) or 340 s (lanes 5–7) and the products were resolved on a 14% PAA/7 M Urea gel. Lane M: 5′-labeled linear 70-mer and 17-mer were loaded as markers. (C) Pol δ (0.1 unit) was incubated in the presence of 12 nM (3′-OH ends) 72:17-mer template-primer under the conditions described in Materials and Methods. Sixteen nanomoles of nM RP-A were added either at the beginning of the reaction or at the indicated times. Reactions were stopped after 5 min and the products were resolved on a 14% PAA/7 M Urea gel. Densitometric analysis of the bands corresponding to the products longer than 90 nt allowed the calculation of their relative amounts. Band intensities (I) were corrected for background and normalized to the total through the equation: relative I = (In/(In-1 + . . . + In-i))/Σ(Ii)i = 1… n, where In is the intensity at the position of interest (n), (In-1 + . . . + In-i) is the sum of the intensities of all bands at positions below n, and Σ(Ii)i = 1… n is the sum of the intensities of all of the bands in the lane. These values were then plotted in dependence of the times of addition of RP-A.