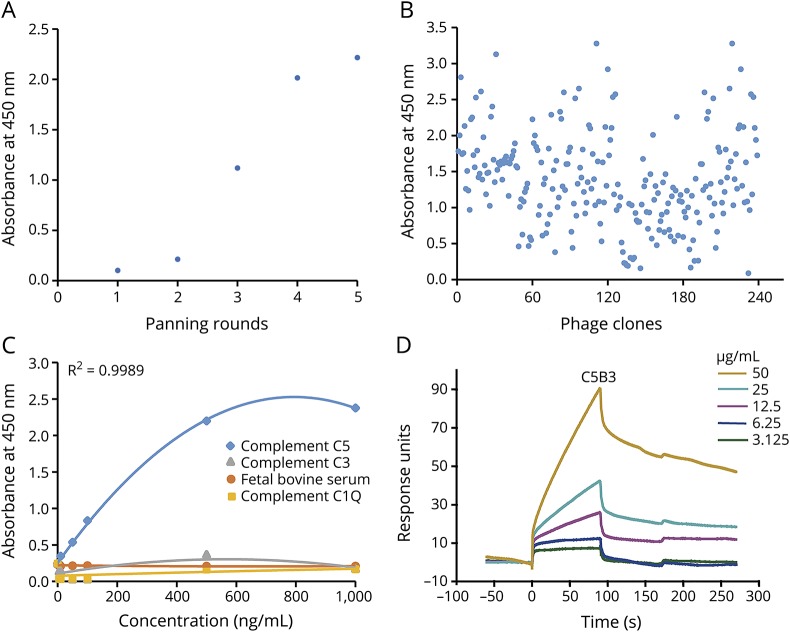
Figure 1. Phage display selected human scFvs against complement C5.
We coated complement C5 on a 96-well streptavidin-coated plate overnight at 4°C, blocked (2% BSA) the wells, and incubated them with phage. Following this, we performed 5 rounds of panning (adsorption-elution-amplification) and identified the phage clones specifically binding to C5 by ELISA. (A) After 5 rounds of panning, the ability of phage antibodies binding to complement C5 gradually increased. (B) As shown by ELISA, 239 randomly selected clones from the fifth round of selection reacted with complement C5. (C) C5B3 binds specifically to complement C5, but had a low affinity for control proteins (i.e., BSA and complements C3 and C1Q). (D) The SPR sensorgram shows concentration-dependent binding of C5B3 to C5. We immobilized complement C5 onto a CM5 sensor chip. We injected C5B3 for 90 seconds over the sensor chip, followed by a 180-second washout period (20 μL/min). BSA = bovine serum albumin; scFvs = single-chain variable fragments; SPR = surface plasmon resonance.