Abstract
Background:
Training in interoceptive awareness is a promising behavioral approach for improving substance use disorder (SUD) treatment. This study examined the longitudinal effects of Mindful Awareness in Body-oriented Therapy (MABT) as an adjunct to women’s SUD treatment. MABT teaches interoceptive awareness skills to promote self-care and emotion regulation.
Methods:
Women in intensive outpatient treatment for SUD at three community clinics were recruited and randomly assigned to one of three study conditions: Treatment as Usual (TAU) + MABT, TAU + Women’s Health Education (WHE), and TAU only. Four assessments were delivered over one year (N =187): baseline, 3, 6 and 12 months to examine primary outcome of percent days abstinent from substance use, and secondary outcomes of emotion dysregulation, craving, psychological distress, mindfulness and interoceptive awareness. Changes in outcomes across time were assessed using multilevel mixed effects linear regression.
Results:
Substance use improved significantly for MABT vs. TAU at 6 months and 12 months. Positive longitudinal effects on secondary outcomes for MABT were evident on respiratory sinus arrhythmia (RSA), a physiological index of emotion regulation; on craving; and on interoceptive awareness skills. Analyses based on participants who completed >75% of the intervention sessions revealed additional immediate significant improvements for MABT vs. TAU and WHE on depressive symptoms and emotion regulation difficulties and longitudinal improvement on mindfulness skills.
Conclusions:
Results show MABT to be efficacious for longitudinal health outcomes to support women’s recovery as an adjunct to community-based SUD treatment.
Keywords: Substance Use Disorder Treatment, Women, Interoception, Randomized Controlled Trial, Complementary Therapies, Emotion Regulation, Respiratory Sinus Arrhythmia, Mindfulness
1. Introduction
Among individuals seeking substance use disorder treatment in publicly-funded programs annually in the United States, 30% are women (Substance Abuse and Mental Health Services Administration, 2016). Compared to men, women in treatment for substance use disorder (SUD) are more likely to have co-occurring mental health disorders and a history of interpersonal trauma, characterized in part by emotion dysregulation known to negatively influence treatment outcomes (Walitzer and Dearing 2006; Greenfield et al., 2007). Emotion dysregulation is characterized by intense emotions, low stress tolerance, experiential avoidance, and a sense of lack of control (Eftekhari et al., 2009). Exposure to childhood abuse followed by later traumatic events in adulthood appears to be strongly linked to emotion dysregulation and lifetime substance use problems (Mandavia et al., 2016), consistent with studies linking lifetime exposure to trauma and substance use (Kevorkian et al., 2015; Lawson et al., 2013). In addition, among individuals with substance use disorders, dysregulated emotion is linked to neurobiological problems of interoceptive processing, known as interoceptive dysfunction (Sinha and Li, 2007; Paulus and Stewart, 2014). Interoception involves the processing of sensations from inside the body (Craig, 2003). Interoception is often not conscious and may negatively impact cognitive control and decision-making processes that underlie patterns of behavior associated with substance use (Verdejo-Garcia et al., 2012; Noel et al., 2013; Paulus and Stewart, 2014). In contrast, interoceptive awareness – the ability to access, attend to, and respond to internal bodily sensations (Farb et al., 2015) – may offset automatic emotional responses and conditioning, and support positive decision-making processes critical for relapse prevention (Paulus and Stewart, 2014). The purpose of this study was to examine interoceptive awareness training for women in treatment, to address the need for more research on adjunctive treatments to improve women’s SUD treatment outcomes (Institute of Medicine, 2010).
Interoceptive awareness training within the context of SUD treatment is a promising behavioral approach to address emotion regulation difficulties in SUD (Price et al., 2012). Interoceptive training is designed to build skills in processing and managing sensory input from the body, including sensing, interpreting, and integrating information about the body (Vaitl, 1996; Khalsa et al., 2018), as a means for reducing emotion dysregulation and the associated substance use behavior patterns that can negatively impact treatment outcomes. Importantly, interoception is recognized as a central mechanism that may underlie mindfulness-based approaches for SUD treatment (Witkiewitz et al., 2013; Garland et al., 2014). Mindfulness-based SUD research has demonstrated reductions in substance use and related health outcomes (Bowen et al., 2009; Witkiewitz and Bowen, 2010; Brewer et al., 2011; Garland et al., 2016). However, mindfulness-based approaches are typically taught in a group context and do not specifically target and develop interoceptive capacity, an identified gap in SUD treatment research (Verdejo-Garcia et al., 2012; Noel et al., 2013; Paulus and Stewart, 2014).
This study examined the efficacy of interoceptive awareness training as an adjunct to intensive outpatient treatment (IOP) to reduce substance use and improve related health outcomes. The active intervention was based on Mindful Awareness in Body-oriented Therapy (MABT), a mindfulness-based approach specifically designed to teach interoceptive awareness and related skills for self-care and emotion regulation. MABT is unique among mindfulness-based approaches in its use of touch to promote and develop the capacity for interoceptive awareness. As a therapeutic approach delivered individually, regulatory responses to sensory experience are assessed, and any difficulty with interoceptive processing is explicitly addressed through a combination of mindfulness instruction and psychoeducation (Price and Hooven, 2018). Our reported immediate pre-post findings highlighted the acquisition of interoceptive awareness skills, improved emotion regulation (self-report and physiological), reduced depression, and perceived benefits of this approach among women in treatment (Price et al., 2018).
This report describes longer-term results from the same randomized controlled trial (RCT). The study included three conditions: treatment as usual (TAU), MABT as an adjunct to treatment as usual (TAU+MABT), and Women’s Health Education (WHE) as an adjunct to TAU and serving as the active control condition. Focusing on outcomes across time and at the 12-month follow-up, the primary study hypothesis was that women randomized to TAU+MABT, compared to TAU and TAU+WHE, would report more days abstinent from substance use. The secondary hypothesis was that TAU+MABT, compared to TAU and TAU+WHE, would result in improved psychological health (reflected in reductions in emotion dysregulation, craving, and psychological symptoms of distress). The third hypothesis was that the intervention process variables of interoceptive awareness and mindfulness skills would improve for TAU+MABT compared to TAU and TAU+WHE.
2. Methods
2.1. Study Design
This NIDA-funded study was a three group, repeated measures, randomized controlled trial. Implemented at three community non-profit outpatient clinics in the Pacific NW, this study included four assessments: pre-intervention at baseline, and post-intervention at 3 months, 6- and 12-month follow-up. Based on a pilot MABT study for women in SUD treatment (Price et al., 2012) and taking into account 25% attrition, the power analysis indicated that a sample size of 225 would be sufficient to detect a moderately small effect size (es = .29), power = .80, alpha = .05, two-tailed. All study procedures were approved by the Institutional Review Board at a large University in the northwestern United States, in accord with the Helsinki Declaration of 1975.
2.2. Procedures
2.2.1. Recruitment and Screening.
Clinic patients were recruited through flyers posted in the clinics for self-referral, and through study description presented in IOP groups by the research coordinator. Interested individuals were screened for eligibility by the research coordinator. Eligibility criteria included: female, over 18 years old, enrolled in the clinic IOP, agreed to forego other manual and mind-body therapies for a period of 3 months (baseline to post-test), fluent in English, able to attend MABT sessions when offered, and available for the duration of the trial. Exclusion criteria included: currently pregnant, untreated psychotic diagnosis or symptoms, and/or cognitive impairment based on a comprehension assessment of the informed consent.
2.2.2. Enrollment and Study Group Assignment.
Eligible individuals were enrolled after providing informed consent for study participation. Following the baseline assessment, participants were randomly assigned to one of the three study conditions: TAU, TAU + MABT (experimental condition), or TAU + WHE (active control condition). Allocation to condition was accomplished using a block random group generator (STATA) to ensure that equivalent number of cases were distributed to TAU, MABT or WHE at each of the three clinics. Toward the end of study recruitment, however, it became necessary to modify the randomization balance (1:1:1) to ensure adequate resources for equivalent and adequate sample sizes in the key experimental and control conditions. The modified proportion of subjects randomized to TAU, MABT, and WHE was 3:3:1, respectively.
2.2.3. Data Collection.
A trained research coordinator at each clinical site administered assessments at baseline, 3, 6 and 12 months. Data were collected at each assessment using: an interview calendar method to gather substance use information; a biochemical assay to screen for recent alcohol and/or drug use, and an online survey to assess secondary self-report outcomes. A set of baseline-only questionnaires was used to collect information regarding demographic and health history (McLellan et al., 1992), economic and legal status, and life-time trauma exposure (Kubany et al., 2000). A BIOPAC MP150 system was used to capture ECG and respiratory signals to assess respiratory sinus arrhythmia (RSA), the physiologic indicator of emotion dysregulation (Beauchaine and Thayer, 2015).
In addition to the assessment procedures outlined above, MABT participants completed follow-up questionnaires at 6 and 12 months to assess their use of MABT skills. We collected data from electronic medical records specific to relapse and treatment adherence. For participants who did not come to the clinic for follow-up assessments, we collected substance use data by phone interview when possible. For this reason, we have data from more study participants on the primary outcome than on the secondary outcomes. Participants were compensated $30 for completion of the baseline assessment, and $40, $50 and $60, respectively, for completion of each subsequent assessment.
2.3. Measures
2.3.1. Primary Outcome.
The primary outcome of days abstinent from substance use was measured using the Time-Line Follow-back interview (TLFB) (Sobell et al., 1996) to assess use of alcohol, illicit drugs, and non-prescription medications including marijuana over the past 90 days. The baseline TLFB included the 90-day period prior to entering SUD treatment. A second dichotomous measure of substance use relapse (0 = no relapse, 1 = relapse) was calculated based on any evidence of relapse from three separate data sources all collected during the same 90 day assessment period, including data from the TLFB, toxicology screen results, and electronic health records.
2.3.2. Secondary outcomes.
Secondary outcomes included measures of emotion regulation (physiological and self-report), craving, psychological distress, mindfulness and interoceptive awareness. Cronbach’s alpha for all self-report measures described below was > .90 (Price et al., 2018). The Difficulties in Emotion Regulation Scale (DERS) (Gratz and Roemer, 2004), is a 36-item measure with total scores ranging from 36 to 180; higher scores corresponded to more difficulties in emotion regulation. Craving was measured using the 5-item Penn Alcohol Craving Scale (PACS) (Flannery et al., 1999), modified to address both alcohol and other drugs. The PTSD Symptom Scale-Self Report (PSS-SR) (Foa et al., 1993), a 17-item questionnaire, was used to assess symptoms of posttraumatic stress based on DSM-IV-TR criteria (American Psychiatric Association, 2000), which also provides screening cut-off values for PTSD (Coffey et al., 1998). The Beck Depression Inventory-II (BDI-II), a 21-item questionnaire, was used to measure severity of depressive symptoms (Beck et al., 1996). Process measures included the Freiburg Mindfulness Inventory (FMI) (Walach et al., 2006) and the Multidimensional Assessment of Interoceptive Awareness (MAIA) (Mehling et al., 2012).
Physiologic data for RSA were recorded using standardized procedures with the BIOPAC MP150 system (BIOPAC Systems Inc., Goleta, CA, USA), with electrodes placed on the torso using a standard spot configuration (Qu et al., 1986). Tonic RSA (resting-state) was measured during a five-minute period, with participants resting quietly. Participants did not eat, drink, or smoke cigarettes for one hour minimum prior to collecting RSA data (Hawkley et al., 2003). RSA was scored in ten 30-second epochs and averaged to produce a mean resting-state RSA value using MindWare HRV 3.0.10 software suite to calculate RSA values (Hayano et al., 1991). Consistent with standard RSA scoring, data were normalized via log transformations within the MindWare algorithm (for further study methods specific to RSA see Crowell et al., 2017).
2.4. Study Conditions and Interventions
All study participants at the three clinical sites were enrolled in IOP, the TAU condition, an abstinence-based treatment program. The program complies with the requirements of the Washington State Code, which regulates chemical dependency treatment, including an admission assessment, development of an individual treatment plan, and recovery-based education for SUD. IOP was offered as group sessions 2–3 times/week for 1.5 hours over 10–14 weeks, with individual counseling sessions once/month, at minimum. A continuing care program focused on relapse prevention, a less-intensive outpatient treatment program, was offered at each of the clinics for those who completed IOP.
Both MABT and WHE, the adjunctive treatments, have manualized protocols composed of 8 weekly 1.5 hour individual sessions, delivered over 8–10 weeks. The MABT intervention is designed to teach interoceptive awareness skills for self-care and emotion regulation using a combination of manual, psychoeducation, and mindfulness approaches. The protocol has three distinct stages for teaching interoceptive awareness and take-home skills. The stages provide an incremental approach to facilitate learning the interoceptive awareness components of Stage 1: identifying body sensations; Stage 2: learning strategies for interoceptive awareness: and Stage 3: developing the capacity to sustain interoceptive awareness as a mindful process to facilitate acceptance, self-understanding/insight, and appraisal of interoceptive experiences (Price and Hooven, 2018). All sessions began with 30 minutes of discussion specific to body awareness and self-care activities in daily life, and then 45 minutes of experiential learning focused on the development and practice of interoceptive awareness skills. The sessions ended with 15 minutes of discussion to review and appraise the session content and identify a take-home practice, based on the session experience, for the interim week.
WHE, an individual health curriculum, was designed to control for time and attention in women’s SUD treatment studies (Miller et al., 1998; Hien et al., 2009). WHE matched the MABT intervention by providing equivalent attention, expectancy of benefit, and an issue-oriented focus without theory-driven techniques (i.e., mindfulness or body awareness). WHE focused on topics such as reproductive health, nutrition, and cardiovascular health. Similar to MABT, all sessions began with a 30-minute discussion related to the health care topic and included 45 minutes of educational films and materials. Sessions ended with a 15 minute discussion of what was learned, and identification of a self-care activity for the interim week based on session materials.
2.5. Sample Characteristics
Participant characteristics are summarized by study group in Table 1. Two hundred seventeen women were recruited for study participation. Approximately 20% of enrolled participants assigned to MABT and WHE withdrew from study participation without attending MABT or WHE sessions, yielding a sample of 187. The sample was primarily white (75%), with 8% Hispanic; the majority reported low socio-economic status; ages ranged from 22–61. The primary substances of use, for which treatment was sought, included stimulants (45%), alcohol (39%), narcotics (24%), marijuana (8%), other opiates or analgesics (6%); 22 % of the participants reported using more than a single “primary” drug. The majority (70%) had previously participated in some form of substance use disorder treatment. Notably, all participants reported having experienced interpersonal trauma, typically characterized by multiple types and events. Correspondingly, 68% scored above the cut-off for PTSD. In addition, 56% of the participants reported court involvement related to their SUD treatment, most commonly linked to Child Protective Services.
Table 1:
Demographic and Baseline Characteristics Based on Intent-to-treat Sample (N=187).
| Category | MABT (n = 74) |
WHE (n = 46) |
TAU (n = 67) |
Total (N= 187) |
|---|---|---|---|---|
| Age (median, range) | 35 (22–61) | 36 (20–59) | 35 (22–57) | 35 (20–61) |
| Race | ||||
| White | 56 (76) | 37 (80) | 47 (70) | 140 (75) |
| Mixed Race | 7 (9) | 4 (9) | 8 (12) | 19 (10) |
| African American | 5 (7) | -- | 4 (6) | 9 (5) |
| Native American | 3 (4) | 3 (7) | 2 (3) | 8 (4) |
| Asian | 1 (1) | 1 (2) | 0 | 2 (1) |
| Hispanic | 5 (7) | 2 (4) | 8 (12) | 15 (8) |
| Highest Education Level | ||||
| 11th grade or less | 16 (22) | 7 (15) | 12 (18) | 35 (19) |
| High school or GED | 28 (38) | 27 (59) | 22 (33) | 77 (41) |
| Two-year college/technical school | 24 (32) | 10 (22) | 24 (36) | 58 (31) |
| College degree (e.g., BA, BS) | 4 (5) | 2 (4) | 7 (10) | 13 (7) |
| Monthly Income | ||||
| No monthly income | 42 (57) | 25 (54) | 28 (42) | 95 (51) |
| Less than $200 | 4 (5) | 7 (15) | 7 (10) | 18 (10) |
| Between $200-$1000 | 18 (24) | 11 (24) | 22 (33) | 51 (27) |
| $1000 or more | 9 (12) | 3 (7) | 9 (13) | 21 (11) |
| Children under 18 years of age | 42 (57) | 31 (67) | 51 (76) | 124 (68) |
| Marital Status | ||||
| Married | 3 (4) | 2 (4) | 6 (9) | 11 (6) |
| Single | 44 (59) | 18 (39) | 31 (46) | 93 (50) |
| Domestic partnership | 2 (3) | 5 (11) | 7 (10) | 14 (7) |
| Divorced | 15 (20) | 11 (24) | 15 (22) | 41 (22) |
| Separated | 7 (9) | 6 (13) | 6 (9) | 19 (10) |
| Other | 2 (3) | 4 (9) | 2 (8) | 8 (4) |
| Currently Employed | 14 (19) | 4 (9) | 8 (12) | 26 (14) |
| Permanent Housing | 39 (53) | 23 (50) | 37 (55) | 99 (53) |
| Health Insurance | ||||
| Medicaid | 56 (56) | 32 (70) | 41 (61) | 129 (69) |
| Medicare | 9 (12) | 6 (13) | 16 (24) | 31 (17) |
| Private | 6 (8) | 4 (9) | 5 (7) | 15 (8) |
| None | 2 (3) | 2 (4) | 2 (3) | 6 (3) |
| Court-Involvement | 37 (50) | 27 (59) | 41 (61) | 105 (56) |
| Interpersonal Trauma History | ||||
| Any reported traumatic event | 74 (100) | 46(100) | 67 (100) | 187 (100) |
| Childhood Sexual Abuse | 42 (57) | 21 (46) | 27 (40) | 90 (48) |
| Childhood Physical Abuse | 29 (39) | 13 (18) | 28 (42) | 70 (37) |
| Adult Physical Assault (stranger) | 28 (38) | 18(39) | 27 (40) | 73 (39) |
| Adult Intimate Partner Violence | 62 (84) | 40 (87) | 52 (78) | 154 (82) |
| Adult Sexual Assault | 29 (39) | 23 (50) | 20 (30) | 72 (39) |
| Adult Stalking | 44 (59) | 28 (61) | 33 (49) | 105 (56) |
| Lifetime Mental Health Services | ||||
| None or < 10 Therapy Sessions | 37 (50) | 24 (52) | 32 (48) | 93 (50) |
| 10–30 Therapy Sessions | 11 (15) | 12 (26) | 16 (24) | 39 (21) |
| > 30 Therapy Sessions | 26 (35) | 10 (22) | 18 (27) | 54 (29) |
Note: Values are number (percentage) unless otherwise indicated. All group comparisons p > .05 at baseline.
Participants had relatively little exposure to mental health care or mind-body approaches. Fifty percent of the total sample reported almost no prior mental health services (< 10 sessions over lifetime, see Table 1). About half (51%) indicated no lifetime mind-body or massage therapy experiences, 32% reported minimal (<10) exposure, 15% moderate (10–30), and only 2% reported high (>30) exposure.
2.6. Treatment Adherence, Study Retention, and Intervention Retention
Examination of adherence to IOP substance use treatment showed that approximately 73% of all study participants completed the IOP program. There was no difference in IOP adherence among the three study groups. Approximately 84% (n = 156) of the full sample continued some form of outpatient treatment after the initial IOP program, either in continuing care (n =135) or by re-enrollment in IOP (n =21); 44% (n =82) completed both IOP and continuing care programs. There were no differences between study groups in either the number who continued in some form of treatment post IOP or completed the continuing care program. At 12 months, about 11% of all study participants (n= 21) were still engaged in outpatient treatment.
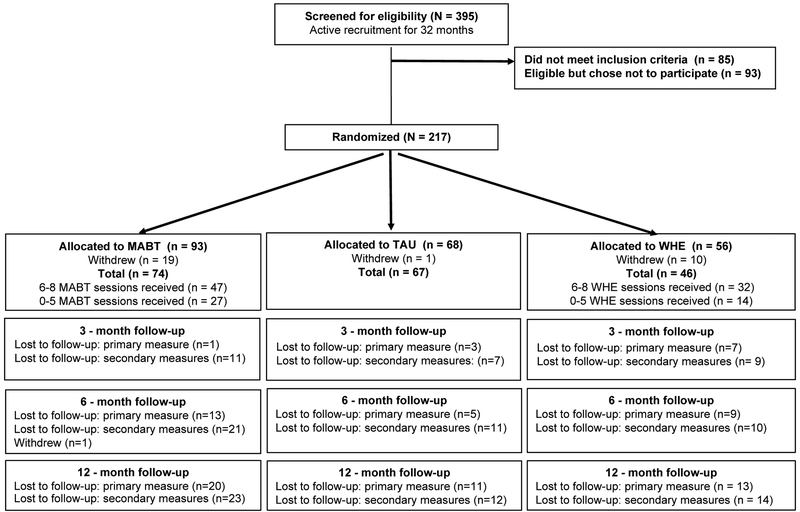
Study retention (i.e., loss to follow-up) among the three study groups varied, with TAU showing the least loss to follow-up, and interventions groups (MABT and WHE) showing similar rate of loss across assessment time-points (see Figure 1). Thus, for the primary outcome of percent days abstinent, TAU loss to follow-up was approximately 7% at 6 months and 16% at 12 months; in contrast, MABT loss was about 18% at 6 months, 27% at 12 months; and WHE loss was 20% at 6 months, 28% at 12 months. For the secondary outcomes, the loss to follow-up pattern was similar across all three study groups. The difference noted for primary outcome vs. secondary outcomes occurred because we were sometimes able to gather substance use data via phone if a participant was unable to come for the full assessment. Intervention retention, defined as attending > 75% of the intervention sessions (N = 146) was 64% for MABT and 70% WHE, see Figure 1.
Figure 1.
Consort Flow Diagram
2.7. Data Analysis
To examine the primary outcome of percent days abstinent from substance use, as well as substance use relapse, we used generalized estimating equations (GEE) models with a logit link, an exchangeable correlation structure, and robust standard errors (Stata 15.1). To manage data that was measured in percentages, we used logit transformations of the outcome variable (total days abstinent) and specified the binomial distribution based on the actual total number of days in the measurement period. This accounted for the number of days in the measurement period that varied across individuals.
With one exception, tests for baseline equivalence among the 3 study groups showed no significant differences for any demographic, sample characteristic, or clinical outcome variables.
The exception was a baseline group difference (Table 2A) in percent of days abstinent during the 3-month period prior to study enrollment and SUD treatment (χ2 = 9.0; p =.01) for MABT (M = 78.0; SD = 26.1), TAU (M = 86.2; SD = 20.7), and WHE (M = 74.3; SD = 27.9). All analyses involving percent days abstinent were adjusted for these differences by including baseline abstinent values as a covariate. We also examined for the potential effects of clinical site and intervention therapist. Inclusion of these variables in the models did not alter the findings or their interpretation for either primary or secondary outcomes.
Table 2:
Primary Outcome: Overall Longitudinal and Focal Group Comparisons for Substance Use, Percent Days Abstinent and Relapse with Intervention Dose Sample (N=146).
| Percent Days | Group Means by Time Mean (SD)c | Wald χ2 Group | Group Differences in Percent Days Abstinent at 3, 6 and 12 Months Mean Difference (95% CI)c | |||||
|---|---|---|---|---|---|---|---|---|
| Abstinenta | MABT | TAU | WHE | p value | Time Point | MABT vs TAU | MABT vs WHE | WHE vs TAU |
| Baseline | 77.8 (26.6) |
86.2 (20.7) |
69.3 (25.7) |
χ2 = 6.95; p = .03 |
||||
| 3 months | 94.1 (18.7) |
90.7 (19.8) |
95.4 (10.1) |
3 months | 5.9 (−1.0 to 12.9) |
−1.8 (−7.0 to 3.3) |
7.8 (1.0 to 14.5)* |
|
| 6 months | 93.1 (15.2) |
82.8 (31.0) |
92.5 (15.9) |
6 months | 11.6 (2.2 to 21.0)* |
0.9 (−6.2 to 8.0) |
10.6 (0.7 to 20.6)* |
|
| 12 months | 91.1 (18.2) |
80.0 (32.0) |
82.2 (30.6) |
12 months | 22.4 (4.5 to 40.3)* |
9.4 (−9.8 to 28.7) |
13.0 (−10.1 to 5.0) |
|
| Relapseb | Number and Percent of Women with Relapse at 3, 6 and 12 Months N/Total N(%) | Wald χ2 Group x Time | Differences in Change for 3 – 6 and 3 – 12 Month Intervals Odds Ratio (95% CI) | |||||
| MABT | TAU | WHE | p value | Interval | MABT vs TAU | MABT vs WHE | WHE vs TAU | |
| 3 months | 14/47 (29.8) |
29/67 (43.3) |
15/32 (46.9) |
χ2
= 2.65; p = .62 |
||||
| 6 months | 19/47 (40.4) |
30/67 (44.8) |
16/32 (50.0) |
3–6 months | 1.51 (0.77 to 2.95) |
1.41 (0.61 to 3.29) |
1.07 (0.50 to 2.28) |
|
| 12 months | 18/47 (38.3) |
34/67 (50.8) |
14/32 (43.8) |
3–12 months | 1.08 (0.49 to 2.40) |
1.65 (0.61 to 4.55) |
.65 (0.24 to 1.74) |
|
Note:
p < .05;
p < .01;
p < .001. Groups: MABT = Mindful Awareness in Body-Oriented Therapy; TAU = Treatment as Usual; WHE = Women’s Health Education.
Estimates for percent days abstinent based on GEE models (logit link) and number of days abstinent adjusted for total days in interval.
Estimates for relapse (0/1) from GEE models (logit link).
c Intervention Dose sample sizes at baseline, 3, 6, and 12 months: MABT (47, 47, 44, 42); TAU (67, 64, 62, 56); WHE (32, 32, 30, 26).
For testing the primary outcome, we used two analytic strategies. First, to examine for overall change across time, we evaluated longitudinal models that included the 3, 6 and 12-month time-points for percent days abstinent and for relapse. This longitudinal analysis included terms for the effect of group and time, and for percent days abstinent were adjusted for baseline values. Second, contrasts were used for focal examination of group differences for percent days abstinent at 3, 6 and 12 months, and for differences in change for substance use relapse for the 3–6 and 3–12 month intervals.
For the secondary outcomes, we used similar analytic strategies. First, we used mixed multilevel models (Stata 15.1, Mixed procedure) to test for longitudinal study group differences across the 12-month study period. The longitudinal models included group and time as categorical variables, a group x time interaction, random intercept, and a random slope for time to account for within-subject correlations. Models were estimated using robust standard errors. Second, we examined for focal differences in change between groups within 3 intervals: from baseline to 3, 6, and 12 months. Group differences for both primary and secondary outcomes were assessed using Wald χ2with significance set at p ≤ .05.
Outcomes were analyzed using both the intervention-dose (ID) sample and the intent-to-treat (ITT) sample. The ID sample (N = 146) included study participants in MABT and WHE who participated in 6 or more (> 75%) of the 8 intervention sessions, and all participants in the TAU group (Figure 1, Consort Flow Diagram). The ITT sample (N = 187) included all subjects for whom data were available for at least one follow-up assessment, and who were fully or partially engaged in the MABT and WHE interventions. The ID findings are summarized in the results and tables below. ITT Tables are available in Supplementary Materials.
3. Results
3.1. Primary Outcome – Days Abstinent from Substance Use
For the ID sample, the longitudinal analysis indicated an intervention effect in which the three groups differed significantly (χ2 = 6.95; p = .03). In the focal between-group contrasts, MABT and WHE showed more abstinent days compared to TAU at 6 months (mean difference = 11.6 (95% CI: 2.2 – 21.0 and 10.6 (95% CI: .7 – 20.6 respectively). At 12 months, only MABT showed more days abstinent compared to TAU, with an adjusted mean difference of 22.4 (95% CI: 4.5 – 40.3). Notably, MABT improvements were maintained from 3–12 months, whereas TAU and WHE showed declines in abstinence days, particularly at 12 months. Fewer women in MABT vs. TAU relapsed over the assessments, although this difference was not statistically significant. No group differences were observed for MABT vs. WHE in the percent of days abstinent or relapse at any assessment time-point.
Results were similar for the ITT sample. The overall longitudinal effect approached significance (χ2 = 5.90; p = 0.052). This result reflected that subsequent to the observed change during the intervention period, relatively modest change occurred from 3 to 12 months for all groups (see Table 2A in Supplementary Materials1). Specifically, for the ITT sample, the focal between-group findings showed significant improvement in percent days abstinent for MABT and WHE compared to TAU at 6 months with an adjusted mean difference of 11.3 (95% CI: 2.1–20.7), and for MABT compared to TAU at 12 months with an adjusted mean difference of 18.9 (95% CI: 0.5–37.2) with an effect size of .32 (Cohen’s d) for a sample size of 110 (MABT n = 54 vs. TAU n = 56).
3.2. Secondary Outcomes
3.2.1. Emotion Dysregulation.
Findings for emotion dysregulation differed markedly between the self-report and physiological measures of this construct. Self-reported emotion dysregulation showed no significant longitudinal differences among the three groups in either the ID (see Table 3) or ITT analyses (see Supplementary Materials1). For the ID sample, focal examination using between group contrasts showed significant reductions in emotion regulation difficulties for MABT vs. TAU from baseline to 3 months (mean difference = −14.2; 95% CI: −23.6 - −4.9), and for MABT vs. WHE from baseline to 3 months (mean difference = −13.8; 95% CI: −24.3 - −3.3). For the ITT sample, MABT improved significantly compared to TAU, but only in the baseline to 3 month interval (mean difference = −10; 95% CI: −18.1- −1.9).
Table 3:
Secondary Outcomes: Overall Longitudinal and Focal Group Comparisons for Emotion Dysregulation with Intervention Dose Sample (N=146).a
| Emotion Dysregulation |
Group Means by Time Mean (SD | Wald χ2 Group x Time | Differences in Change at 3, 6 and 12 Months Mean Difference in Change (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MABT | TAU | WHE | p value | Interval | MABT vs TAU | MABT vs WHE | WHE vs TAU | ||
| Difficulties in Emotion Dysregulation (DERS)b | |||||||||
| Baseline | 88.1 (28.2) |
83.8 (27.3) |
90.7 (30.0) |
χ2 = 11.45; p = .08 |
|||||
| 3 months | 74.1 (23.8) |
83.6 (27.5) |
89.9 (27.6) |
Baseline - 3 months | −14.2 (−23.6 to −4.9)** |
−13.8 (−24.3 to −3 3)** |
−0.4 (−9.2 to 8.3) |
||
| 6 months | 74.4 (25.9) |
75.9 (25.0) |
82.8 (25.4) |
Baseline - 6 months | −8.3 (−17.3 to 0.8) |
−7.6 (−18.3 to 3.2) |
−0.7 (−9.9 to 8.5) |
||
| 12 months | 76.5 (29:4) |
75.6 (21.9) |
81.1 (23.1) |
Baseline - 12 months | −5.2 (−16.9 to 6.4) |
−3.7 (−18.8 to 11.5) |
−1.5 (−15.1 to 12.0) |
||
| Respiratory Sinus Arrhythmia (RSA)c | |||||||||
| Baseline | 5.35 (2.02) |
5.52 (1.39) |
5.45 (1.70) |
χ2 = 68.96; p < .001 |
|||||
| 3 months | 5.78 (1.57) |
5.45 (1.52) |
5.38 (1.52) |
Baseline - 3 months | 0.53 (0.36 to 0.70)*** |
0.57 (0.38 to 0.77)*** |
−0.4 (−0.23 to 0.15) |
||
| 6 months | 5.64 (1.69) |
5.19 (1.454) |
5.33 (1.94) |
Baseline - 6 months | 0.29 (0.10 to 0.47)** |
0.19 (−0.02 to 0.41) |
0.09 (−0.11 to 0.30) |
||
| 12 months | 5.785 (1.95) |
5.52 (1.351) |
4.95 (1.92) |
Baseline - 12 months | .44 (0.25 to 0.64)*** |
.72 (0.48 to 0.95)*** |
−0.27 (−0.50 to −0.05)** |
||
Note:
p < .05;
p < .01;
p < .001. Groups: MABT=Mindful Awareness in Body-Oriented Therapy; TAU=Treatment as Usual; WHE=Women’s Health Education.
Estimates from multilevel mixed effects models.
Intervention dose sample sizes at baseline, 3, 6, and 12 months: MABT (47, 46, 40, 41); TAU (66, 59, 55, 54); WHE (32, 32, 30, 26).
Intervention dose sample sizes at baseline, 3, 6, and 12 months: MABT (47, 45, 34, 31); TAU (66, 52, 47, 43); WHE (32, 29, 24, 18).
In contrast, for RSA, the overall longitudinal effects were significant for both the ID and ITT samples (Wald χ2 = 68.96 and 68.16, p < .001, respectively). For the ID sample (see Table 3), the focal analysis revealed a greater increase in RSA for MABT vs. TAU and WHE from baseline to 3 months (mean difference = 0.53, 95% CI: 0.36 – 0.70 and .57, CI: 0.38 – 0.77, respectively), for MABT vs. TAU at 6 months (mean difference = 0.29: 95% CI: 0.10 −0.47), and for MABT vs. TAU and WHE from baseline to 12 months (mean difference = 0.44, 95% CI: 0.25 – 0.64 and 0.72, CI: 0.48 – 0.95, respectively), with WHE also showing significantly lower RSA vs. TAU at 12 months. The ITT results were similar (see Table 3A in Supplementary Materials), with MABT showing significantly greater change in RSA vs. TAU and WHE at both 3 and 12 months.
3.2.2. Craving.
For the ID sample, the overall longitudinal test (Table 4) indicated significant reductions in craving across the 12 months (χ2 = 13.7, p =.03), which was similar to the ITT sample results (χ2 = 12.7; p = .047) (Table 4 A in Supplementary Materials). Focal analyses of group differences in change for the ID and ITT samples were also similar, both indicating significant improvement for MABT vs. TAU at 6 and 12 months. Craving was significantly less in the ID sample for MABT vs. TAU at 3 months (mean difference = −3.2; 95% CI: −0.7), at 6 months (mean difference = −5.5; 95% CI: −8.5 - −2.5) and 12-month follow-up (mean difference = −4.0; 95% CI: −7.4- −0.5). No focal differences in craving were observed for MABT vs. WHE.
Table 4:
Secondary Outcomes: Overall Longitudinal and Focal Group Comparisons for Craving and Psychological Distress with Intervention Dose Sample (N=146).a
| Craving and Psychological Distress |
Group Means by Time Mean (SD)b | Wald χ2 Group x Time | Differences in Change from Baseline to 3, 6 and 12 Months Mean Difference in Change (95% CI)b | |||||
|---|---|---|---|---|---|---|---|---|
| MABT | TAU | WHE | p value | Interval | MABT vs TAU | MABT vs WHE | WHE vs TAU | |
| Craving | ||||||||
| Baseline | 13.6 (8.0) |
10.7 (7.5) |
14.5 (9.2) |
χ2 = 13.69; p = .03 |
||||
| 3 months | 9.4 (6.4) |
9.3 (8.1) |
11.1 (8.7) |
Baseline - 3 months | −3.2 (−5.8 to −0.7)** |
−0.9 (−4.0 to 2.3) |
−2.3 (−5.6 to 0.9) |
|
| 6 months | 8.5 (7.7) |
11.3 (8.5) |
10.3 (8.1) |
Baseline - 6 months | −5.5 (−8.5 to −2.5)*** |
−0.6 (−4.2 to 3.0) |
−4.9 (−8.5 to −1.3)** |
|
| 12 months | 9.0 (8.2) |
10.0 (8.6) |
11.0 (9.0) |
Baseline - 12 months | −4.0 (−7.4 to −0.5)* |
−1.2 (−5.4 to 3.0) |
−2.8 (−6.9 to 1.3) |
|
| Trauma Symptoms (PSS-SR) | ||||||||
| Baseline | 20.6 (11.9) |
18.5 (12.4) |
20.8 (13.8) |
χ2 = 6.26; p =.40 |
||||
| 3 months | 11.8 (11.0) |
13.9 (11.8) |
17.3 (13.2) |
Baseline - 3 months | −4.0 (−8.9 to 0.8) |
−5.2 (−11.2 to 0.9) |
1.1 (−4.3 to 6.5) |
|
| 6 months | 14.2 (12.2) |
11.6 (12.4) |
13.6 (11.8) |
Baseline - 6 months | 0.1 (−5.8 to 6.0) |
0.3 (−5.5 to 6.1) |
−0.2 (−5.4 to 5.0) |
|
| 12 months | 14.0 (13.4) |
11.6 (11.1) |
15.0 (12.3) |
Baseline - 12 months | −0.0 (−5.6 to 5.5) |
−1.3 (−7.7 to 5.1) |
1.3 (−4.1 to 6.7) |
|
| Depression Symptoms (BDI) | ||||||||
| Baseline | 16.8 (11.1) |
15.3 (10.5) |
15.5 (11.8) |
χ2 = 10.11; p = .12 |
||||
| 3 months | 11.2 (8.5) |
13.9 (11.4) |
15.3 (12.4) |
Baseline - 3 months | −4.5 (−8.3 to −0.7)* |
−5.6 (−9.7 to −1.5)** |
1.1 (−2.8 to 4.9) |
|
| 6 months | 10.7 (8.5) |
11.6 (9.4) |
12.7 (10.1) |
Baseline - 6 months | −3.4 (−6.9 to 0.2) |
−3.3 (−7.5 to 0.8) |
−0.0 (.4.0 to 4.0) |
|
| 12 months | 12.3 (10.6) | 11.5 (9.8) |
13.5 (11.2) |
Baseline - 12 months | −1.6 (−6.2 to 3.0) | −2.7 (−8.9 to 3.4) | 1.1 (−4.4 to 6.6) |
|
Note:
p < .05;
p < .01;
p < .001. Abbreviations: MABT=Mindful Awareness in Body-Oriented Therapy; TAU=Treatment as Usual; WHE=Women’s Health Education. Measures: Penn Alcohol Craving Scale (PACS), PSS-SR: PTSD Symptom Scale–Self-Report; BDI: Beck Depression Inventory.
Estimates from multilevel mixed effects models.
Intervention dose sample sizes at baseline, 3, 6, and 12 months: MABT (47, 46, 40, 41); TAU (66, 59, 55, 54); WHE (32, 32, 30, 26).
3.2.3. Psychological Distress.
The longitudinal tests of psychological distress (trauma and depression symptoms) revealed no significant differences among the three study groups from baseline to 12 months for either the ID or ITT samples. The ID focal analysis, however, showed significant reduction in depression symptoms for MABT vs. TAU (mean difference = −4.5; 95% CI: −8.3 - −0.7) and for WHE (mean difference = −5.6; 95% CI: −9.7 - −1.5) from baseline to 3 months (see Table 4). No group differences were observed at any time-point in the ITT analyses.
3.2.4. Mindfulness and Interoceptive Awareness Skills.
For mindfulness skills, the overall longitudinal effect in the ID sample was statistically significant (18.14; p = .006). Focal between group comparisons for the ID sample (see Table 5) revealed significant improvements in mindfulness skills in MABT vs. TAU and WHE from baseline to 3 months (mean difference = 4.9; 95% CI: 1.2–8.7 and 7.6; CI: 2.7–12.4, respectively), and from baseline to 6 months (mean difference = 5.0; 95% CI: 1.8–8.2 and 6.9; CI: 2.6–11.2, respectively). At 12 months, only MABT vs. WHE showed differences. In contrast, the ITT longitudinal analysis for mindfulness skills was not significant. Focal analysis of mindfulness skills showed greater change for MABT vs. TAU from baseline to 6-months (mean difference = 3.7; 95% CI: 0.7 – 4.7), see Table 5A in Supplementary Materials.
Table 5:
Secondary Outcomes: Overall Longitudinal and Focal Group Comparisons for Mindfulness and Interoceptive Awareness Skills with Intervention Dose Sample (N = 146).a
| Mindfulness and Interoceptive Awareness |
Group Means by Time Mean (SD)b | Wald χ2 Group x Time | Differences in Change from Baseline to 3, 6 and 12 Months Mean Difference in Change (95% CI)b | |||||
|---|---|---|---|---|---|---|---|---|
| MABT | TAU | WHE | p value | Interval | MABT vs TAU | MABT vs WHE | WHE vs TAU | |
| Mindfulness Skills (FMQ) | ||||||||
| Baseline | 34.8 (9.5) |
35.6 (9.9) |
37.8 (8.9) |
χ2 = 18.14; p = .006 |
||||
| 3 months | 39.8 (8.1) |
36.1 (10.3) |
35.5 (10.7) |
Baseline - 3 months | (1.2 to 8.7)** | 7.6 (2.7 to 12.4)** |
−2.6 (−6.9 to 1.6) |
|
| 6 months | 40.0 (9.3) |
36.5 (10.2) |
36.5 (10.6) |
Baseline - 6 months | 5.0 (1.8 to 8.2)** |
6.9 (2.6 to 11.2)** |
−1.9 (−6.0 to 2.2) |
|
| 12 months | 38.5 (9.7) |
38.1 (9.4) |
35.9 (8.6) |
Baseline - 12 months | 1.9 (−2.4 to 6.2) |
6.3 (1.4 to 11.3)* |
−4.4 (−8.7 to −0.1)* |
|
| Interoceptive Awareness (MAIA) | ||||||||
| Baseline | 2.6 (0.9) |
2.5 (0.9) |
2.7 (0.9) |
χ2 = 34.77; p < .001 |
||||
| 3 months | 3.5 (0.8) |
2.6 (0.9) |
2.6 (0.8) |
Baseline - 3 months | 0.8 (0.5 to 1.2)*** |
1.0 (0.6 to 1.4)*** |
−0.2 (−0.5 to 0.1) |
|
| 6 months | 3.3 (0.8) |
2.7 (0.9) |
2.6 (0.9) |
Baseline - 6 months | 0.4 (0.1 to 0.8)** |
0.8 (0.4 to 1.1)*** |
−0.3 (−0.6 to −0.1)* |
|
| 12 months | 3.2 (0.8) |
2.8 (0.9) |
2.8 (0.9) |
Baseline - 12 months | 0.3 (−0.1 to 0.7) |
0.5 (0.0 to 0.9)* |
−0.1 (−0.5 to 0.2) |
|
Note:
p < .05;
p < .01;
p < .001. Groups: MABT=Mindful Awareness in Body-Oriented Therapy; TAU=Treatment as Usual; WHE=Women’s Health Education. Measures: FMQ: Freiburg Mindfulness Inventory; MAIA: Multidimensional Assessment of Interoceptive Awareness.
Estimates from multilevel mixed effects models.
Intervention dose sample sizes at baseline, 3, 6, and 12 months: MABT(47, 46, 40, 41); TAU(66, 59, 55, 54); WHE(32, 32, 30, 26).
For interoceptive awareness skills, a significant overall longitudinal effect between groups was observed across time for the ID (43.77; p = <.001) and ITT samples (22.69; p = <.001). Between-group focal comparisons showed that MABT improved significantly compared to TAU and WHE from baseline to 3 months (mean difference = 0.8; 95% CI: 0.5–1.2 and 0.6; CI: 0.6 −1.4, respectively), and from baseline to 6 months (mean difference = 0.4; CI: 0.1–0.8 and 0.8; CI: 0.4 – 1.1, respectively), see Table 5. The ITT sample results were similar, showing significant improvement for MABT vs. TAU and WHE at 3 and 6 months, but no differences between groups at 12 months (see Table 5A in Supplementary Materials4).
3.3. MABT Skill Use During Follow-Up Study Period
At 6-month follow-up, 53 MABT participants completed the assessment, and of these 40/53 (75.5%) indicated use of skills learned in the intervention over the past 3 months, and 35/53 (66%) reported weekly use of MABT skills: once/week for 8/53 (15%), several times/week for 17/53 (32%), and daily use for 10/53 (19%). At 12-month follow-up, 51 MABT participants completed the assessment, and of these 40/51 (78.4%) indicated use of MABT in the past 3 months and 35/51 (68.6%) reported continued weekly use of MABT skills: once/week for 17/51 (33.3%), 1–3 times/week for 7/51 (13.7%), and daily use for 11/51 (21.6%).
4. Discussion
This is, to our knowledge, the first study to evaluate the efficacy of interoceptive awareness training to reduce substance use and improve related health outcomes as an adjunct to women’s SUD treatment. The results highlight the significant longitudinal reduction in days abstinent from substance use for women enrolled in MABT + TAU compared to TAU at 6 and 12 month follow-up. WHE +TAU also showed a significant increase in abstinent days compared to TAU at 6 months, suggesting that time and attention played an important role for abstinence during SUD treatment, when the majority of the sample was still engaged in treatment. However, the longer-term effect at 12 months for MABT, once SUD treatment supports were no longer in place, emphasizes the positive effect of interoceptive awareness training to reduce risk of relapse to substance use. Notably, all study groups reported a relatively high (≥ 80%) percent days abstinent at 3, 6 and 12 months (Table 2). This level of abstinence is similar to prior mindfulness SUD treatment studies (Bowen et al., 2009; Bowen et al., 2014) and is higher than typically reported in the SUD treatment literature (Laudet et al., 2007; Byrne and Petry, 2011). In our study, the overall high number of abstinent days is likely due to participant engagement in ongoing SUD treatment over much of the year-long study period. Studies show that women are retained longer in treatment when involved in the criminal justice system (Substance Abuse and Mental Health Services Administration, 2013), of which the majority of this sample was and may reflect, as expressed by many in this sample, the motivation to maintain or regain custody of their children.
Among the psychological health indicators, RSA showed the most marked longitudinal change for those who received MABT, with significant improvement for MABT compared to both TAU and WHE study groups across all three assessment time-points in the ID analysis. Notably, these findings support the use of RSA as a biomarker of emotion dysregulation in SUD treatment research (Kelly and Bardo, 2016; Price and Crowell, 2017; D’Souza et al., 2018). While change in self-reported difficulties in emotion regulation did not show as strong or lasting an effect, there was a similar pattern across the physiologic and self-report indices of emotion regulation showing maintained improvement from 3–12 months for MABT. In contrast, TAU and WHE showed a drop in mean scores during the same time period. Reductions in craving were consistent with the substance use and RSA outcomes, with MABT, compared to TAU, showing significant longitudinal reductions in craving at 3, 6 and 12 month follow-up in the ID analysis. These combined longer-term MABT effects in substance use, emotion regulation and craving are notable given that MABT is a relatively brief adjunct treatment for this highly distressed sample. This may be explained by participants’ improved ability to discern interoceptive cues (physical and emotional) and to engage in self-care behaviors (such as directly processing and managing vs. avoiding emotional distress or triggers that promote craving), in support of their recovery. These findings are consistent with emerging neuroscience models and research (Verdejo-Garcia et al., 2012; Noel et al., 2013; Paulus and Stewart, 2014) and mindfulness frameworks (Garland et al., 2014; Tang et al., 2016) that pinpoint the relevance of interoception for improved regulation and positive behavior change among those with SUD.
Symptoms of psychological distress (trauma and depression) improved for all participants from baseline to 3 and 6 months, likely reflecting the SUD treatment effects. There were no significant group differences, although participants in MABT showed the largest drop in trauma-related symptoms at 3 and 6 months, reflected in the proportion of women in MABT (44% and 50% respectively) compared with TAU (39% and 45%) and WHE (29% and 39%) whose scores shifted from above to below the screening cutoff for PTSD. Notable group differences were observed in the ID analysis, which showed significant reductions in depression compared to TAU and WHE at three months (i.e., pre-post intervention). The improvements in depression and self-reported emotion regulation difficulties distinct in the ID analysis underscore the benefits of receiving the full MABT intervention, and point to a need for a longer MABT intervention period and/or booster sessions, and possibly additional supports for this high stress, high trauma-exposed, low-income population. In addition, the ID analysis findings highlight the stronger effect of MABT in general (including the other outcomes of substance use, RSA, craving, mindfulness and interoceptive awareness skills), demonstrating that the full intervention period is critical for the development and integration of interoceptive awareness skills for improved immediate and long-term health outcomes.
The significant and maintained improvements in interoceptive awareness for MABT compared to TAU and WHE from baseline to 3 and 6 months (and marginally at 12 months) in both the ID and ITT analyses, and similarly, for mindfulness skills in the ID analysis, reflect the skills learned in MABT. Importantly, MABT participants also reported continued use of MABT skills through 12 months, demonstrating the perceived helpfulness of this intervention and the feasibility of integrating these skills into daily life. These findings are consistent with earlier immediate results revealing that MABT skills facilitate sensory awareness, the ability to manage stressors, and support relapse prevention (Price et al., 2018). The longer-term use of MABT skills observed in this study corresponds with findings from other MABT studies (Price et al., 2012; Price and Smith-DiJulio, 2016). Taken together these clinical trials, conducted with women from very different populations, provide compelling support for reinforcing SUD treatment with specific and systematized interoceptive awareness training to promote well-being and reduce risk of relapse.
Limitations as well as strengths of this study warrant discussion. Implemented in clinics that served a primarily low SES population, we do not know if these results generalize to women with higher SES. Prior results from a MABT feasibility study based on a higher SES population indicated a longer-term positive impact on psychological symptoms (Price et al., 2012), suggesting that the length of intervention period may need to be modified depending on internal resources and external supports of the population being served. We also do not know if these results generalize to men in treatment, and this is a question for future MABT research. We also do not know how loss to follow-up, lower in the control (TAU) group than in the intervention groups, may have affected the outcomes. Study strengths include implementation in community-based treatment to teach a mind-body practice to an underserved population, a large sample such that there was sufficient statistical power to detect meaningful effects, use of both objective and self-report indices of emotion regulation, and use of an active control group carefully matched to MABT to control for time and attention, and long-term follow-up. Notably, the inclusion of an active control (WHE) indicated that time and attention may explain the immediate effect on substance use and to 6 months on craving; however, these improvements were not maintained to 12 months, and no other study outcomes were improved in the short term or longitudinally in response to WHE. As each WHE session was a stand-alone information session, with no incremental skill-building components or personal growth orientation, the results also show that intervention dose was not a factor in WHE outcomes.
5. Conclusions
The study results show MABT to be efficacious for longitudinal health outcomes to support women’s recovery; specifically, reductions in substance use and craving, and substantially increased RSA and interoceptive awareness skills (and mindfulness skills in ID analysis) as an adjunct to community-based SUD treatment. As the first large study of interoceptive awareness training in the context of SUD treatment, these results are consistent with prior neuroscience models and research and mindfulness frameworks that suggest the importance of gaining interoceptive skills for emotion regulation and relapse prevention. The ID and ITT analyses showed similar results; nonetheless, the stronger effect of MABT on the majority of measures as demonstrated in the ID analysis emphasizes the importance of receiving the full MABT intervention to support SUD treatment outcomes. Importantly, as an adjunctive study with a highly traumatized and distressed sample, the results point to remarkable longitudinal gains in response to a relatively short intervention. In conclusion, these study findings, in conjunction with earlier MABT studies, indicate that MABT may be an effective adjunct to reinforce and improve women’s SUD treatment.
Supplementary Material
Highlights:
Significant longitudinal improvement in substance use
Significant longitudinal improvement in RSA, craving, and interoceptive awareness skills
Intervention dose effects on symptoms of depression and emotion dysregulation
Use of Mindful Awareness in Body-oriented skills reported through 12 months
Acknowledgements
The authors would like to acknowledge Susan Graham for her project management and Sunny Chieh Cheng, PhD for her contributions to data management.
Role of Funding Source
This study was funded by R01 DA033324 from the National Institute on Drug Abuse, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflict declared.
References
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association, Washington, DC. [Google Scholar]
- Beauchaine TP, Thayer JF, 2015. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int. J. Psychophysiol 98, 338–350. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer A, Brown G, 1996. BDI-II, Beck depression inventory: manual. Psychological Corp., Harcourt Brace, Boston. [Google Scholar]
- Bowen S, Chawla N, Collins S, Witkiewitz K, Hsu S, Grow J, Clifasefi S, Garner M, Douglass A, Larimer M, Marlatt A, 2009. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Subst. Abus 30, 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Witkiewitz K, Clifasefi S, Grow J, Chawla N, Hsu S, Carroll H, Harrop E, Collins S, Lustyk M, Larimer M, 2014. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA Psych. 71, 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Mallik S, Babuscio T, Nich C, Johnson H, Deleone C, Minnix-Cotton C, Byrne S, Kober H, Weinstein A, Carroll K, Rounsaville B, 2011. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend. 119, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne SA, Petry N, 2011. Concurrent alcohol dependence among methadone-maintained cocaine abusers is associated with greater abstinence. Exp. Clin. Psychopharmacol 19, 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Dansky B, Falsetti S, Saladin M, Brady K, 1998. Screening for PTSD in a substance abuse sample: psychometric properties of a modified version of the PTSD Symptom Scale Self-Report. Posttraumatic stress disorder. J. Trauma Stress 11, 393–399. [DOI] [PubMed] [Google Scholar]
- Craig AD, 2003. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol 13, 500–505. [DOI] [PubMed] [Google Scholar]
- Crowell S, Price C, Puzia M, Yaptangco M, Cheng S, 2017. Emotion dysregulation and autonomic responses to film, rumination, and body awareness: Extending psychophysiological research to a naturalistic clinical setting and a chemically dependent female sample. Psychophysiology 54, 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza J, Wardle M, Green C, Lane S, Schmitz J Vujanovic A, 2018. Resting heart rate variability: Exploring associations with symptom severity in adults with substance use disorders and posttraumatic stress. J. Dual Diagn 11, 1–6. DOI: 10.1080/15504263.2018.1526431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari A, Zoellner LA, Vigil SA, 2009. Patterns of emotion regulation and psychopathology. Anxiety Stress Coping 22, 571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Daubenmier J, Price C, Gard T, Kerr C, Dunn B, Klein AC, Paulus MP, Mehling WE, 2015. Interoception, contemplative practice and health. Front. Psychol 6, 763 DOI: 10.3389/fpsyg.2015.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery B, Volpicelli J, Pettinati H, 1999. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol. Clin. Exp. Res 23, 1289–1295. [PubMed] [Google Scholar]
- Foa E, Riggs D, Dancu C, Rothbaum B, 1993. Reliability and validity of a brief instrument for assessing Posttraumatic-Stress-Disorder. J. Trauma. Stress 6, 459–473. [Google Scholar]
- Garland EL, Froeliger B, Howard M, 2014. Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Front. Psychiatry 4, 173 DOI: 10.3389/fpsyt.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Roberts-Lewis A, Tronnier C, Graves R, Kelley K, 2016. Mindfulness-Oriented Recovery Enhancement versus CBT for co-occurring substance dependence, traumatic stress, and psychiatric disorders: Proximal outcomes from a pragmatic randomized trial. Behav. Res. Ther 77, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Roemer L, 2004. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. J. Psychopathol. Behav. Assess 26, 41–54. [Google Scholar]
- Greenfield S Brooks A, Gordon S, Green C, Kropp F, McHugh R, Lincoln M, Hien D, Miele G, 2007. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend. 86, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Burleson MH, Berntson GG, Cacioppo JT, 2003. Loneliness in everyday life: Cardiovascular activity, psychosocial context, and health behaviors. J. Pers. Soc. Psychol 85, 105–120. [DOI] [PubMed] [Google Scholar]
- Hayano J, Sakakibara Y, Yamada A, Yamada M, Mukai S, Fujinami T, Yokoyama K, Watanabe Y, Takata K, 1991. Accuracy of assessment of cardiac vagal tone by heart rate variability in normal subjects. Am. J. Cardiol 67, 199–204. [DOI] [PubMed] [Google Scholar]
- Hien D Wells E, Jiang H, Suarez-Morales L, Campbell A, Cohen L, Miele G, Killeen T, Brigham G, Zhang Y, Hansen C, Hodgkins C, Hatch-Maillette M, Brown C, Kulaga A, Kristman-Valente A, Chu M, Sage R, Robinson J, Liu D, Nunes E, 2009. Multisite randomized trial of behavioral interventions for women with co-occurring PTSD and substance use disorders. J. Consult Clin. Psychol 77, 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee on Women’s Health Research, 2010. Women’s health research: Progress, pitfalls, and promise. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Kelly TH, Bardo MT, 2016. Emotion regulation and drug abuse: Implications for prevention and treatment. Drug Alcohol Depend. 163, Suppl. 1, S1–S2. [DOI] [PubMed] [Google Scholar]
- Kevorkian S, Bonn-Miller M, Belendiuk K, Carney D, Roberson-Nay R, Berenz E, 2015. Associates among trauma, posttraumatic stress disorder, cannabis use, and cannabis use disorder in a nationally representative epidemiologic sample. Psychol. Addict. Behav 29, 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa S, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, Feusner JD, Garfinkel SN, Lane RD, Mehling WE, Meuret AE, Nemeroff CB, Oppenheimer S, Petzschner FH, Pollatos O, Rhudy JL, Schramm LP, Simmons WK, Stein MB, Stephan KE, Van den Bergh O, Van Diest I, von Leupoldt A, Paulus MP, 2018. Interoception and mental health: A roadmap. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubany E, Haynes S, Leisen M, Owens J, Kaplan A, Watson S, Burns K, 2000. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychol. Assess 12, 210–224. [DOI] [PubMed] [Google Scholar]
- Laudet A, Stanick V, Sands B, 2007. An exploration of the effect of on-site 12-step meetings on post-treatment outcomes among polysubstance-dependent outpatient clients. Eval. Rev 31, 613–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson K, Back S, Hartwell K, Maria M, Brady K, 2013. A comparison of trauma profiles among individuals with prescription opioid, nicotine, or cocaine dependence. Am. J. Addict 22, 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan A, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M, 1992. The fifth edition of the Addiction Severity Index. J. Subst. Abuse Treat 9, 199–213. [DOI] [PubMed] [Google Scholar]
- Mehling W, Price C, Daubenmier J, Acree M, Bartmess E, Stewart A, 2012. The multidimensional assessment of interoceptive awareness (MAIA). PLoS One 7, e48230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Pagan D, Tross S, 1998. Women’s health education, in Peer Activism for Female Partners of Injection Drug Users. Columbia University, New York. [Google Scholar]
- Mandavia A, Robinson G, Bradley B, Ressler K, Powers A, 2016. Exposure to childhood abuse and later substance use: Indirect effects of emotion dysregulation and exposure to trauma. J. Trauma. Stress 29, 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel X, Brevers D, Bechara A, 2013. A neurocognitive approach to understanding the neurobiology of addiction. Curr. Opin. Neurobiol 23, 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL, 2014. Interoception and drug addiction. Neuropharm. 76, 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Wells EA, Donovan DM, Rue T, 2012. Mindful awareness in body-oriented therapy as an adjunct to women’s substance use disorder treatment: a pilot feasibility study. J. Subst. Abuse Treat 43, 94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Crowell S, 2016. Respiratory Sinus Arrhythmia as a potential measure in substance use treatment-outcome studies. Addiction 111, 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Hooven C, 2018. Interoceptive awareness skills for emotion regulation: Theory and approach of Mindful Awareness in Body-Oriented Therapy (MABT). Front. Psychol 9, 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Smith-DiJulio K, 2016. Interoceptive awareness is important for relapse prevention: Perceptions of women who received mindful body awareness in substance use disorder treatment. J. Addict. Nurs 27, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Thompson EA, Crowell SE, Pike K, Cheng SC, Parent S, Hooven C, 2018. Immediate effects of interoceptive awareness training through Mindful Awareness in Body-oriented Therapy (MABT) for women in substance use disorder treatment. Subst. Abus 20, 1–14. DOI: 10.1080/08897077.2018.1488335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu MH, Zhang YJ, Webster JG, Tompkins WJ, 1986. Motion artifact from spot and band electrodes during impedance cardiography. IEEE Trans. Biomed. Eng 33, 1029–1036. DOI: 10.1109/TBME.1986.325869. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li C, 2007. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 26, 25–31. [DOI] [PubMed] [Google Scholar]
- Sobell L,M, Sobell G, Buchan P, Cleland I, Fedoroff, Leo G, 1996. The reliability of the Timeline Followback method applied to drug, cigarette, and cannabis use The 30th Annual Meeting of the Association for Advancement of Behavior Therapy, New York. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2018. Center for Behavioral Health Statistics and Quality. Treatment Episode Data Set (TEDS): 2016. Admissions to and Discharges from Publicly Funded Substance Use Treatment. Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2013. Substance Abuse Treatment: Addressing the specific needs of women Treatment Improvement Protocol (TIP) Series, No. 51. HHS Publication No. (SMA) 13–4426. Rockville, MD. [Google Scholar]
- Tang YY, Tang RX, Posner MI, 2016. Mindfulness meditation improves emotion regulation and reduces drug abuse. Drug Alcohol Depend. 163, S13–S18. [DOI] [PubMed] [Google Scholar]
- Vaitl D, 1996. Interoception. Biol. Psychol 42, 1–27. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn B, 2012. The role of interoception in addiction: a critical review. Neurosci. Biobehav. Rev 36, 1857–1869. [DOI] [PubMed] [Google Scholar]
- Walach H, Buchheld N, Buttenmüller V, Kleinknecht N, Schmidt S, 2006. Measuring mindfulness—the Freiburg Mindfulness Inventory (FMI). Pers. Individ. Dif 40, 1543–1555. [Google Scholar]
- Walitzer KS, Dearing RL, 2006. Gender differences in alcohol and substance use relapse. Clin. Psychol. Rev 26, 128–148. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S, 2010. Depression, craving, and substance use following a randomized trial of Mindfulness-Based Relapse Prevention. J. Consult. Clin. Psych 78, 362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Lustyk M, Bowen S, 2013. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol. Addict. Behav 27, 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.