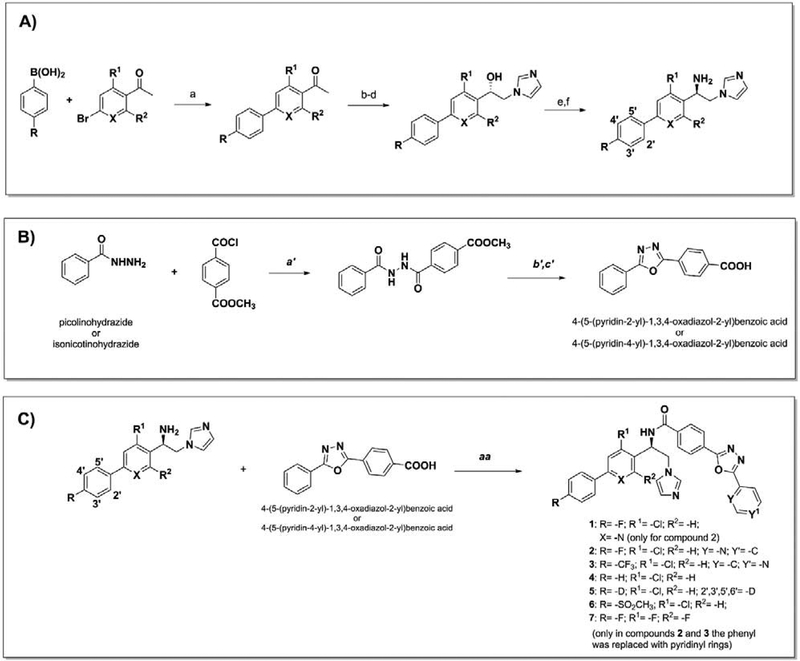
Scheme 1. A. Synthesis of 1–7.
Reagent and conditions: a) Pd(PPh3)4, Na2CO3, toluene/EtOH/H2O, reflux, 3 h, under argon. Yield 80% to quantitative. b) CuBr2, CHCl3/EtOAc, reflux 2 h. Yield 80%. c) Imidazole, DMF, 0 °C, 2 h. Yield 50–80%. d) RuCl(p-cymene)[(R,R)-Ts-DPEN], CH2Cl2 TEA, HCO2H, 40 °C, 26 h. Yield 80–90%. e) DMF, DPPA, DBU, 0 °C rising to room temperature, overnight, under argon. Yield 50–70%. f) THF, LiAlH4, room temperature overnight. Yield >99%. B. a′) THF, 6 h, at room temperature. b′) POCl3, reflux overnight, 85 °C. c′) LiOH·H2O, THF, CH3OH, H2O (3:1:1, v/v/v,), 3 h at room temperature. Yield 70–90%. C. aa) CH2Cl2, EDC/DMAP, room temperature, overnight, under argon. Yield 30–80%. Enantiomeric excess 97–99%.