Figure 1.
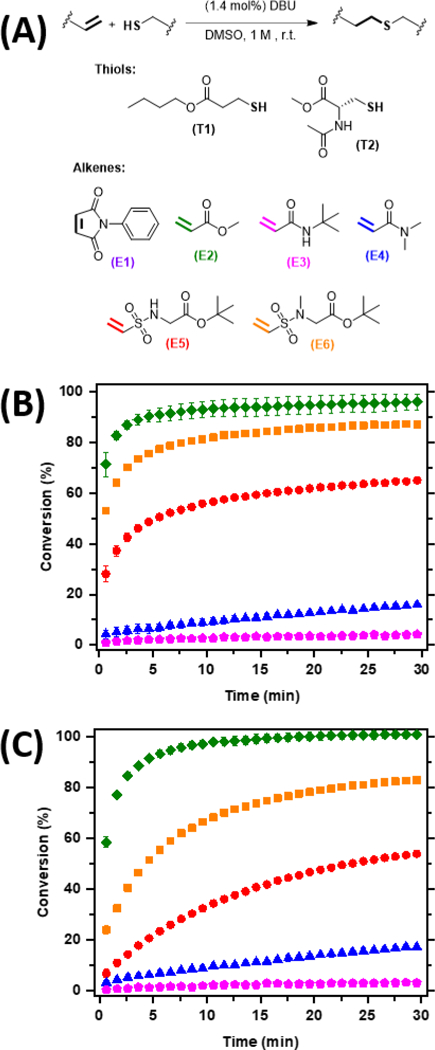
Reaction kinetics of various thiols and electron poor alkenes. A) The general thiol-Michael reaction scheme, and the model thiols and alkenes used in this study. B) Reaction kinetics were monitored at room temperature over 30 minutes using FTIR spectroscopy by tracking the disappearance of the thiol-peak between 2484 and 2545 cm−1. 1 eq of the thiol, T1, was reacted with 1 eq of each of the alkenes, E2 (green, filled diamond), E3 (magenta, filled pentagon), E4 (blue, filled triangle), E5 (red, filled circle), and E6 (orange, filled square) in the presence of DBU (1.4 mol%) in DMSO. (C) Similarly, 1 eq of the thiol, T2, was reacted with 1 eq of each alkene, E2 (green, filled diamond), E3 (magenta, filled pentagon), E4 (blue, filled triangle), E5 (red, filled circle), and E6 (orange, filled square). E1 is not shown as it reached full conversion with both T1 and T2 within the first 30 s of the experiment.
