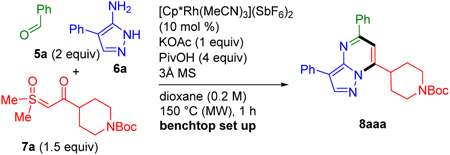
Table 1.
Reaction Parameters for Annulation to Pyrazolopyrimidine 8a
| entry | Variation | Yield %b |
|---|---|---|
| 1 | None | 75 |
| 2 | [Cp*RhCl2]2 (5%) | 61 |
| 3 | no Rh | 0 |
| 4 | NaOAc instead of KOAc | 60 |
| 5 | no KOAc | 39 |
| 6 | no PivOH | 27 |
| 7 | 160 °C | 73 |
| 8 | chlorobenzene as solvent | 57 |
| 9 | ylide 7a (1.1 equiv) | 67 |
| 10 | [Cp*IrCl2]2 (5%) and AgSbF6 (20%) | 28 |
| 11 | [Cp*Co(MeCN)3](SbF6)2 (10%) | 21 |
| 12 | 0.1 M | 62 |
| 13 | 0.4 M | 85 (82)c |
| 14 | 0.4 M, no sieves | 75 |
| 15 | 0.4 M, [Cp*Rh(MeCN)3](SbF6)2 (5%) | 77 |
| 16 | 0.4 M, [Cp*Rh(MeCN)3](SbF6)2 (2.5%) | 38 |
| 17 | 0.4 M, conventional heating, 100 °C, 16 h | 82 |
Conditions: 5a (0.20 mmol), 6a (0.10 mmol), 7a (0.15 mmol)
Yield determined by 1H-NMR relative to 1,3,5-trimethoxybenzene as external standard.
Isolated yield of a 0.30 mmol scale (see Scheme 2).
