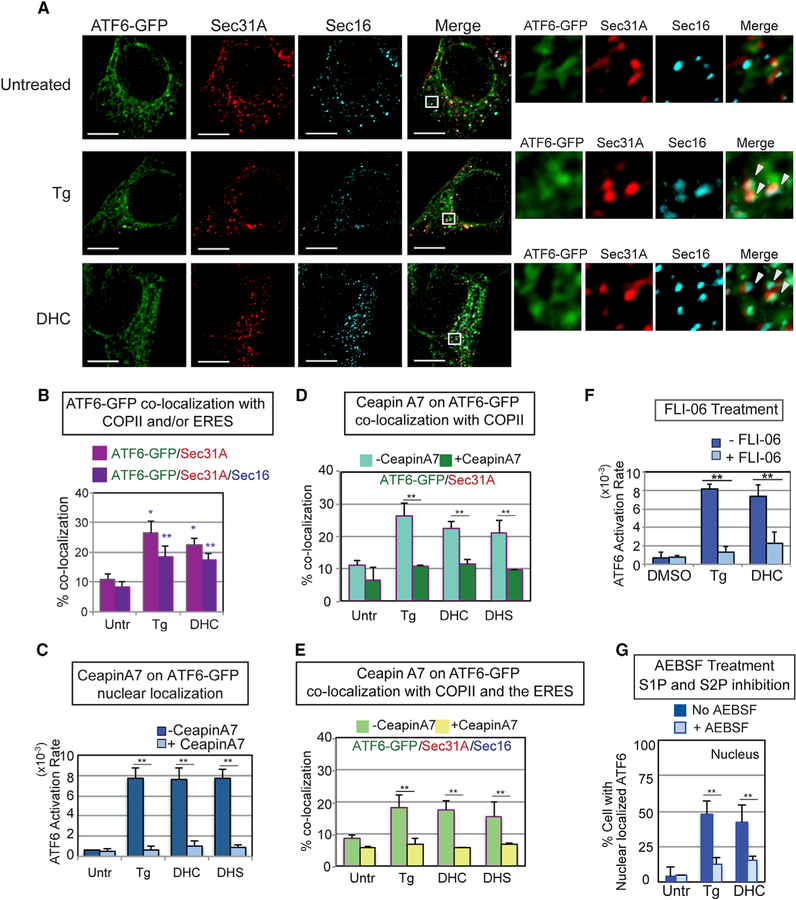
Figure 4. DHS/DHC Activation of ATF6 Requires Transport to the Golgi via COP II Vesicles and S1P/S2P Cleavage of the ATF6 Transmembrane Domain.
(A) DHC treatment resulted in co-localization of ATF6-GFP with Sec31A, a COPII vesicle component, and Sec16, an ER exit site (ERES) component, to an extent similar to ATF6-GFP in Tg-treated cells (arrowheads indicate co-localization of all three molecules). Following treatment of HEK293 cells with Tg or DHC for 60 min, immunofluorescence was done with anti-Sec31A and anti-Sec16 antibodies. Representative images of cells treated with Tg, DHC, or untreated are shown, with high magnification views of the white-boxed areas at the right. ATF6-GFP in green, Sec31A in red, and Sec16 in blue. Scale bar, 10 µm. (B) Percent co-localization of ATF6-GFP with Sec31A (magenta) and Sec16 (dark purple) is shown. See STAR Methods for a detailed description of the quantification. Each co-localization experiment was repeated at least three independent times. (C) Ceapin A7, which blocks Tg activation of ATF6 as defined by nuclear localization, inhibits DHC and DHS activation of ATF6 to a similar extent. (D and E) Ceapin A7 inhibits co-localization of DHC-, DHS-, and Tg-induced ATF6-GFP with Sec31A (D) and with both Sec31A and Sec16 (E) upon treatment with ATF6. (F) DHC activation of ATF6 requires transport to the Golgi. Pretreatment of HEK293 cells with FLI-06, an inhibitor of COPII transport, blocked ATF6-GFP activation by Tg and DHC. (G) ATF6 activation by either Tg or DHC is similarly blocked by AEBSF, a known chemical inhibitor of the membrane-bound proteases S1P and S2P. In (C)–(G), **p < 0.01 and *p < 0.05 using unpaired two-tailed t tests comparing untreated and treated samples.