Table 4.
Substrate Scope of Trifluoromethyl Imines with α,β-Unsaturated Lactonesa
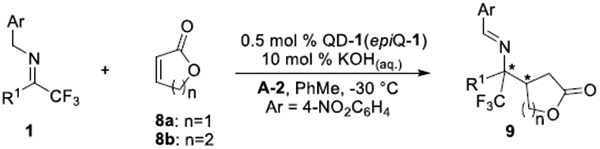
| entry | R1; 1 | 8 | t (h) | Yield of 9 (%)b | ee of 9 (%)c |
|---|---|---|---|---|---|
| 1d | Me; 1A | 8a | 1 | 93 (94); 9Aa | 99 (98) |
| 2d | n-Bu; 1C | 8a | 3 | 89 (90); 9Ca | 99 (98) |
| 3e | Cy; 1G | 8a | 5 | 84 (82); 9Ga | 99 (98) |
| 4 | PhCH=CH; 1K | 8a | 2 | 75 (71); 9Ka | 99 (98) |
| 5 | Ph; 1L | 8a | 2 | 70 (69); 9La | >99 (>99) |
| 6 | 3-OMeC6H4; 1M | 8a | 2 | 55 (51); 9Ma | >99 (>99) |
| 7 | 4-ClC6H4; 1N | 8a | 2 | 50 (48); 9Na | >99 (>99) |
| 8e | Me; 1A | 8b | 3 | 86 (91); 9Ab | 94 (91) |
Unless noted, reactions were performed with 1 (0.2 mmol), 8 (0.4 mmol), aqueous KOH solution (2.2 μL, 50wt %, 10 mol %), A-2 (10 mol % for entries 1–3 and 8, 50 mol % for entries 47) in PhMe (2.0 mL) with catalyst QD-1 (0.5 mmol %); results in parentheses were obtained from reactions catalyzed by epiQ-1 (0.5 mol %) in same conditions..
Isolated yield of 9, dr of 9 >95/5.
Determined by HPLC analysis.
1.0 mol % catalysts were employed.
2.0 mol % catalysts were employed.
