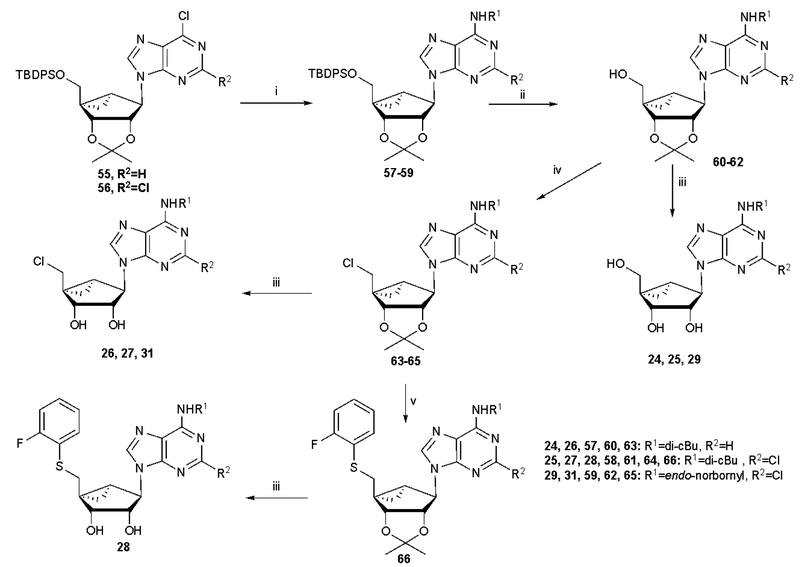
Scheme 1.
Synthesis of (N)-methanocarba derivatives bearing typical A1AR-enhancing groups at the N6 and C2 positions with and optionally substituted at the 5′ position with chloro or an aryl thioether. Reagents and conditions: (i) R1NH2, DIPEA, 2-propanol, rt; (ii) TBAF, THF, rt; (iii) 10% TFA, MeOH, 70 oC; (iv) SOCl2, pyridine, CH3CN, −5 oC-rt (v) 2-F-PhSH, NaH, DMF, 0 oC-rt.