Figure 2. Identification of 1 as a novel inhibitor of SHP2.
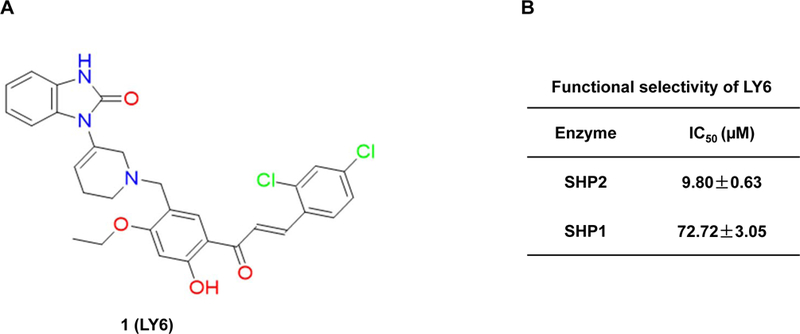
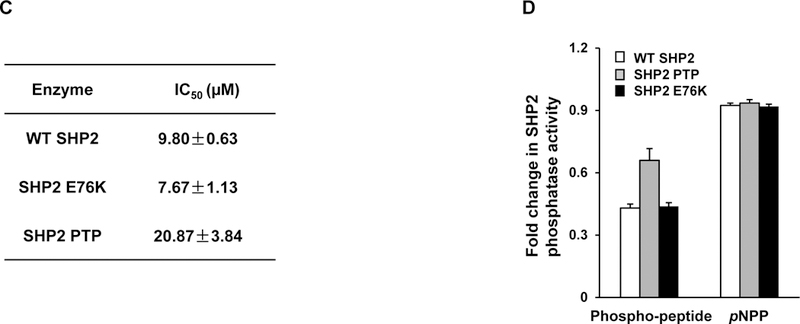
(A) Chemical structure of 1. (B) Phosphatase assays were carried out using full length SHP2 or SHP1 as the enzyme and a phospho-peptide as the substrate in the presence of various concentrations of 1, as described in Experimental Section. For IC50 determinations, six concentrations of 1 were tested. Results shown are the mean ± SD of three independent experiments. (C) Phosphatase activities of full length WT SHP2, full length SHP2 E76K, and the SHP2 PTP domain were assessed in the presence of 1 at various concentrations as above. IC50 values were then determined. Experiments were repeated independently three times, similar results were obtained in each experiment. Results shown are the mean ± SD of triplicates from one experiment. (D) Phosphatase activities of full length WT SHP2, full length SHP2 E76K, and the SHP2 PTP domain were determined in the presence of 1 (10 μM) using a phospho-peptide or pNPP as the substrate, as detailed in Experimental Section Experiments were repeated independently three times, similar results were obtained in each experiment. Results shown are the mean ± SD of triplicates from one experiment.
