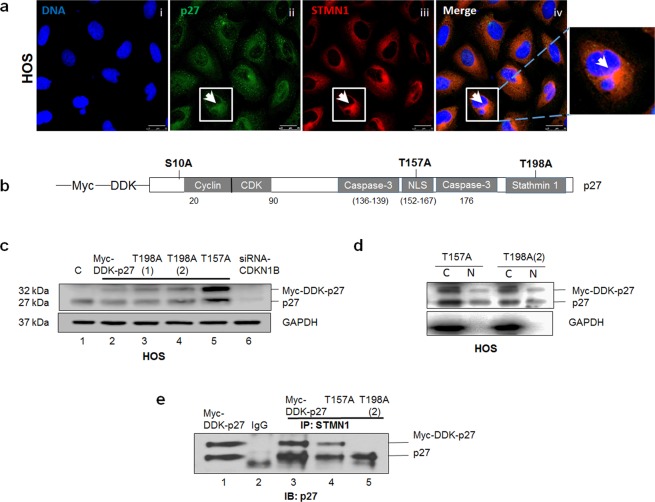
Figure 2.
The interaction between p27 and STMN1 is dependent on T198 phosphorylation. (a) HOS cells were subjected to immunofluorescence staining using anti-p27 and anti-STMN1 antibodies. p27 is colored green; STMN1 is colored red; nuclei were stained with Hoechst dye and are colored blue. Amplification of boxed area highlights the merged staining of cytoplasmic p27 and STMN1 for a single cell. Magnification is 63x; scale bars = 25 μm. (b) Schematic representation of selected phosphorylation sites (S10, T157, T198), protein binding domains (cyclin/CDK, caspase-3, stathmin1) and the nuclear localization sequence (NLS) within the p27 amino acid sequence. (c) HOS cells expressing recombinant wild-type p27 (Myc-DDK-p27), T157A and T198A mutant p27 protein, and attenuated p27 (siRNACDKN1B) were lysed and examined by immunoblot analysis with anti-p27 antibody. Recombinant wt p27 protein is represented at 32 kDa and endogenous p27 is at 27 kDa. GAPDH was the protein loading control. (d) Nuclear and cytoplasmic lysates were extracted from HOS cells expressing T157A and T198A mutant p27 protein. Lysates were examined by immunoblot analysis with anti-p27 antibody. GAPDH was the cytosolic marker. (e) HOS cells expressing Myc-DDK-p27, T157A and T198A mutant p27 protein were lysed and subjected to immunoprecipitation with anti-STMN1 antibody and p27 detection with anti-p27 antibody. The results are representative of three independent experiments.