Figure 11.
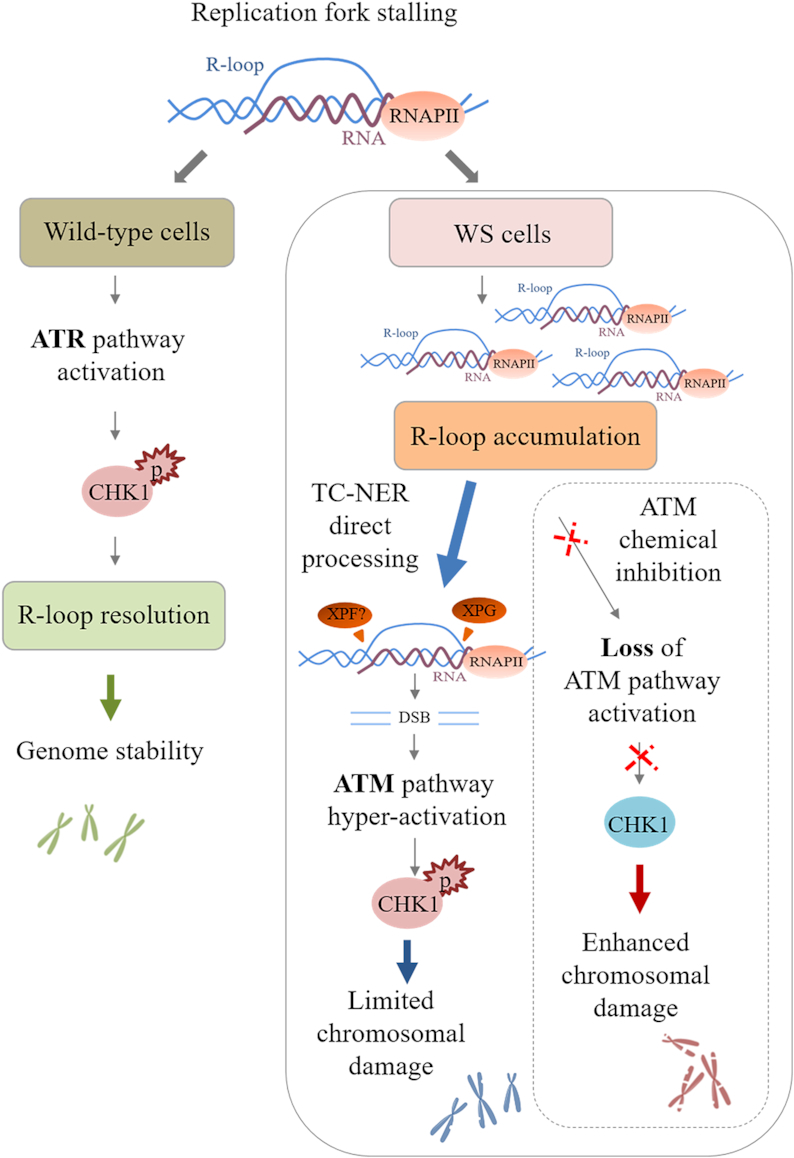
Model for a role of ATM pathway in limiting genome instability in WS cells. After replication fork stalling, the presence of WRN leads to a correct ATR-dependent CHK1 activation that ensures R-loop resolution and thus genome stability. In the absence of WRN, although an aberrant accumulation of R-loops is detected, a direct processing of these structures by the endonuclease XPG induces transient DSB formation, leading to ATM pathway hyper-activation. Under these conditions, active ATM mediates CHK1 phosphorylation that is crucial to limit chromosomal damage in WS cells. However, when ATM kinase is chemically inhibited, the failure to respond to R-loop-associated DNA damage enhances chromosomal damage, which is already elevated in WS cells.
