Table 1.
Optimization of reaction conditions.
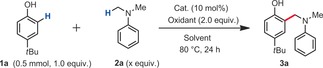
| Entry | x | Catalyst | Oxidant | Solvent | Yield [%][a] |
|---|---|---|---|---|---|
| 1 | 1.6 | L1CuCl2 | DTBP | Cumene 1.5 mL | 20 |
| 2 | 4.8 | L1CuCl2 | DTBP | Cumene 1.5 mL | 38 |
| 3 | 9.5 | L1CuCl2 | DTBP | Cumene 1.5 mL | 55 |
| 4 | 9.5 | L1CuCl2 | DTBP | Cumene 1.0 mL | 60(62) |
| 5 | 9.5 | L1CuCl2 | DTBP | Cumene 0.5 mL | 55 |
| 6 | 9.5 | L1CuCl2 | DTBP | Benzene 1.0 mL | 34 |
| 7 | 9.5 | L1CuCl2 | DTBP | Toluene 1.0 mL | 58 |
| 8 | 9.5 | L1CuCl2 | DTBP | t Bu‐benzene 1.0 mL | 29 |
| 9 | 9.5 | L1CuCl2 | TBHP | Cumene 1.0 mL | trace |
| 10 | 9.5 | L1CuCl2 | TBPB | Cumene 1.0 mL | trace |
| 11 | 9.5 | Cu(TC) [c] | DTBP | Cumene 1.0 mL | 34 |
| 12[b] | 9.5 | CuF2+L1 | DTBP | Cumene 1.0 mL | 42 |
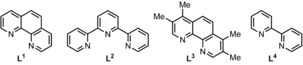
| 13[b] | 9.5 | CuCl2+L2 | DTBP | Cumene 1.0 mL | 12 |
| 14[b] | 9.5 | CuCl2+L3 | DTBP | Cumene 1.0 mL | 29 |
| 15[b] | 9.5 | CuCl2+L4 | DTBP | Cumene 1.0 mL | 12 |
| |||||
[a] The yield was determined by 1H NMR analysis of the crude reaction mixture using 1,3,5‐trimethoxybenzene as an internal standard. [b] The amount of ligand was 15 mol %. [c] thiophene‐2‐carboxylate.
