Figure 6.
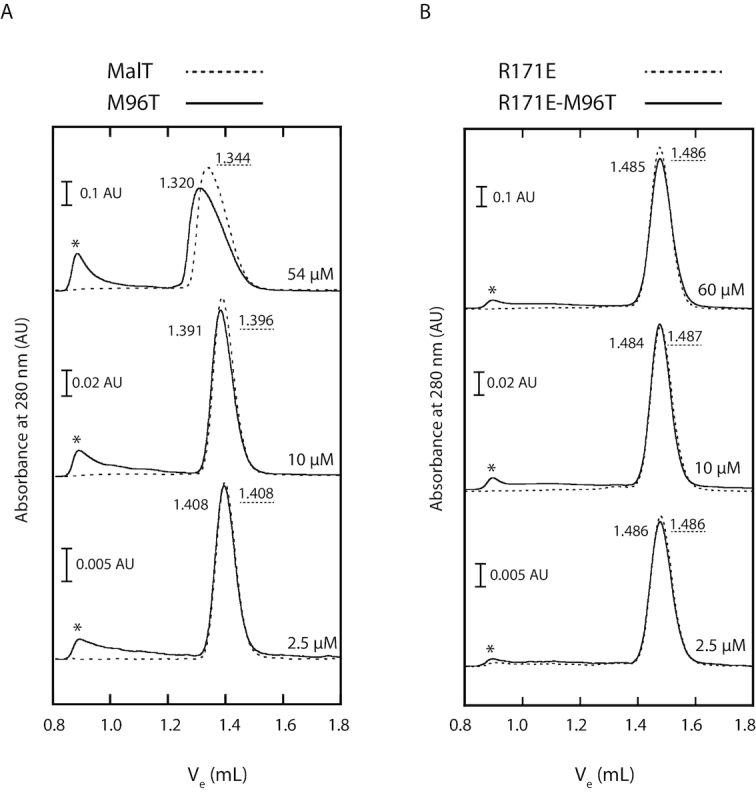
The M96T substitution increases native MalT-ADP multimerization in the absence of maltotriose. (A) After a 10-min preincubation in a Tris–HCl buffer (50 mM, pH 8.0) containing 10% sucrose, 0.033 M K3 citrate, 10 mM Mg acetate, 0.1 mM EDTA, 1 mM dithiothreitol and 0.1 mM ADP, MalT or MalTM96T were injected at the indicated concentration on a Superdex 200 column equilibrated with the same buffer. The MalTM96T concentration was adjusted to account for the presence of aggregates (eluting as peak in the void volume, marked by a star), probably due to partial opening of the protein and release of the bound ADP during the purification in the absence of nucleotide. Note the different scales. The 54 and 2.5 μM experiments were repeated three times by alternating the two proteins on the same column. For these experiments, the mean elution volume (ml) is indicated (with dotted underline for MalT). Variations in the elution volumes between the three repeats did not exceed 0.002 ml. (B) MalTR171E (elution volumes with dotted underline) or MalTM96T,R171E was preincubated and injected as above on a Superdex 200 column with the same characteristics but with a shifted calibration curve (see Supplementary Figure S12) due to a longer time of use. On that column, the elution volume for a wt MalT monomer (in the same conditions, protein concentration 10 μM) was 1.484 ml. MalTM96T,R171E aggregates are also marked by a star. The 60 μM experiments were repeated twice and the variations did not exceed 0.003 ml.
