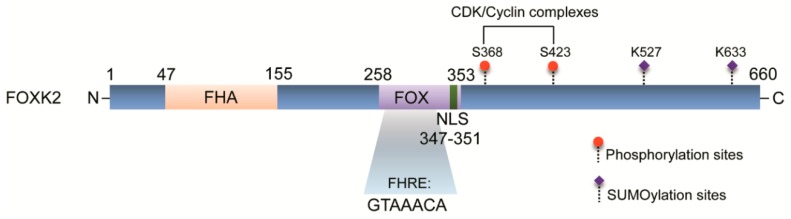
Figure 1.
The domain structure of FOXK2 protein. FOXK2 is composed of a forkhead-associated domain (FHA) localized towards the amino terminus and a highly conserved forkhead DNA-binding domain (FOX) localized towards the carboxy-terminal end of the protein. The FOX domain displays a nuclear localization signal (NLS) and mediates FOXK2 binding to consensus sequences with a GTAAACA core motif. The post-translational modifications (PTM) for FOXK2 are shown, where serines (S) are targets for phosphorylation by cyclin-dependent kinases (CDK) and lysines (K), for SUMOylation. Amino-acids sites which are targets for PTM are depicted above the scheme. FHRE, forkhead responsive elements.