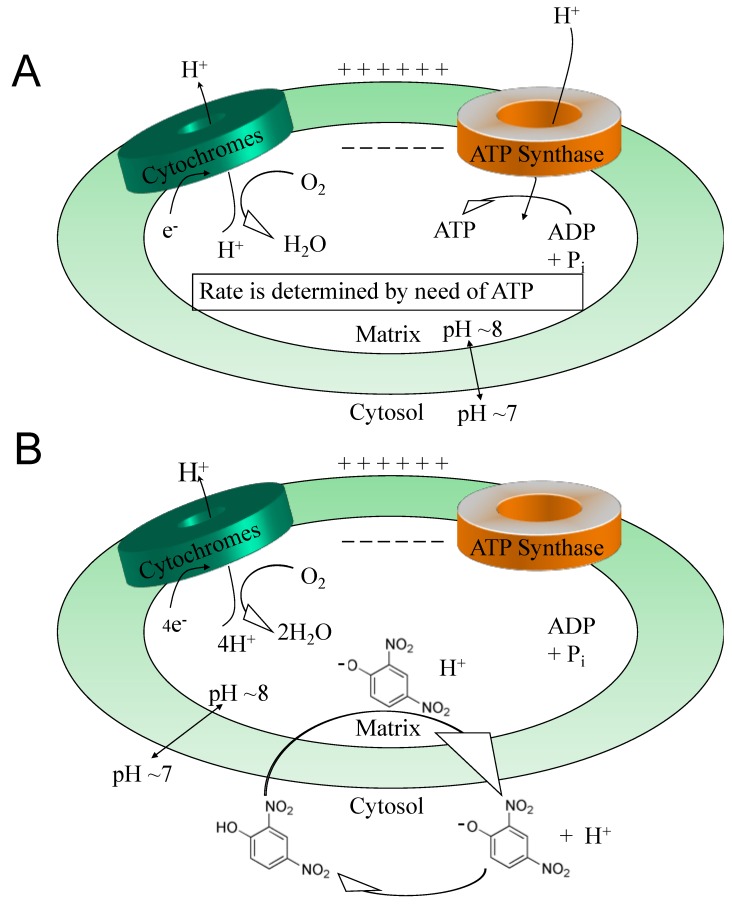
Figure 2.
Coupling vs. uncoupling. Maintaining the proton gradient across the mitochondrial matrix and cytosol involves the pumping of protons out of the matrix via cytochromes I, III and IV. (A) The coupling of a proton (hydrogen or H+) transfer to the synthesis of ATP is a result of H+ returning through ATP synthase, causing rotation and subsequent phosphorylation of ADP, thereby yielding an ATP molecule. (B) This mechanism is circumvented in the case of chemical uncoupling (i.e., entry of protons without phosphorylation to produce ATP). The proton transfer into the matrix is on a carrier, a weak acid molecule (i.e., 2,4-dinitrophenol) with a unique dissociable proton (H+) due to the two NO2 “electron withdrawing” groups. Outside of the mitochondria in the acidic environment of the cytosol, DNP is in the protonated neutral form, but attracted to the basic environment of the matrix. Upon entering the matrix, DNP releases the dissociable proton (H+). Now in the negatively charged anionic form, DNP is attracted to the acidic environment of the cytosolic space, therefore returning to the cytosol to become reprotonated and the cycle starts over again. All mitochondrial systems remain functional but are accelerated. Reprinted by permission from Springer, Diabetologia (2011) 54:237–244, Targeting Energy Expenditure via Fuel Switching and Beyond, J.G. Geisler [2].