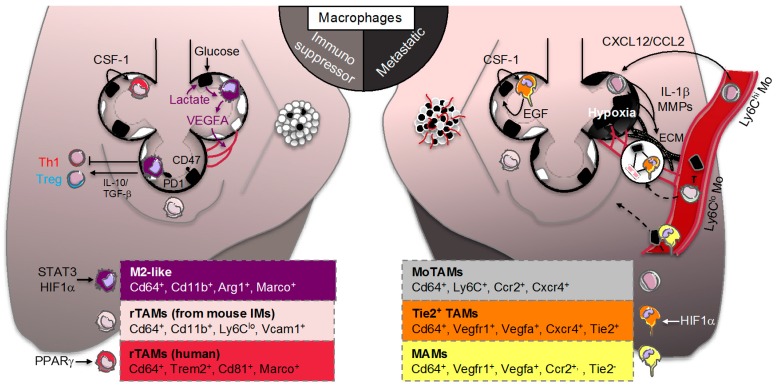
Figure 2.
Macrophage effector functions as part of the 7th hallmark of cancer. Right panel: In established tumors, tumor-associated macrophages (TAMs) are the major part of the immune infiltrate that constitutes the tumor microenvironment (TME). Malignant cells produce the colony stimulating factor 1 (CSF-1), which participates to the conversion of tissue-resident macrophages into resident-TAMs (rTAMs). Their origin and cell surface makers may differ between mice and humans, with PPARγ being highly expressed in human rTAMs. Tumor cells also produce lactate through anaerobic glycolysis referred as the “Warburg effect” that can feed cancer cells in a cell-autonomous fashion for proliferation or act in a paracrine fashion to stabilize the hypoxia-inducible factor 1α (HIF1α) and promote a non-classical “M2-like” macrophage polarization. The signal transducer and activator of transcription 3 (STAT3) is another key transcription factor of M2 polarization. These M2-like macrophages participate to the tumor growth through at least 4 mechanisms: (1) secretion of the angiogenic vascular endothelial growth factor A (VEGFA), (2) expression of the immune checkpoint programmed death-1 (PD-1), (3) defect in recognizing and phagocytosing CD47-expressing tumor cells and (4) immunosuppression through inhibition of Th1 helper cells (Th1) and recruitment of regulatory T cells (Treg). Left panel: TAMs are also involved in more chaotic metastatic tumors. A feed-forward loop between CSF-1-expressing tumor cells and EGF-expressing TAMs contributes to intensive proliferation and oxygen consumption leading to a hypoxic environment. Tumor cells also secrete chemokine ligands such as CXCL12 and CCL2, involved in the recruitment into the tumor site of newly monocyte-derived TAMs (MoTAMs) from circulating Ly6Chi monocytes contributing to the expansion of the tumor and the hypoxic niche. Hypoxia within tumor nest alters tumor cells and surrounding MoTAMs promoting extracellular matrix (ECM) remodeling through secretion of IL-1β and metalloproteases (MMPs). This remodeling favors the “angiogenic switch”. A population of Tie2+ TAMs, which most likely derives from a subpopulation of circulating Ly6Clo monocytes, is located within the tumor vasculature interacting with mammalian-enabled (MENA)-expressing tumor cells and endothelial cells to further promote angiogenesis and create a metastatic environment. Circulating Ly6Clo monocytes also scavenge tumor materials to prevent tumor invasion whereas metastasis-associated macrophages (MAMs) allow the extravasation of tumor cells into the lung.