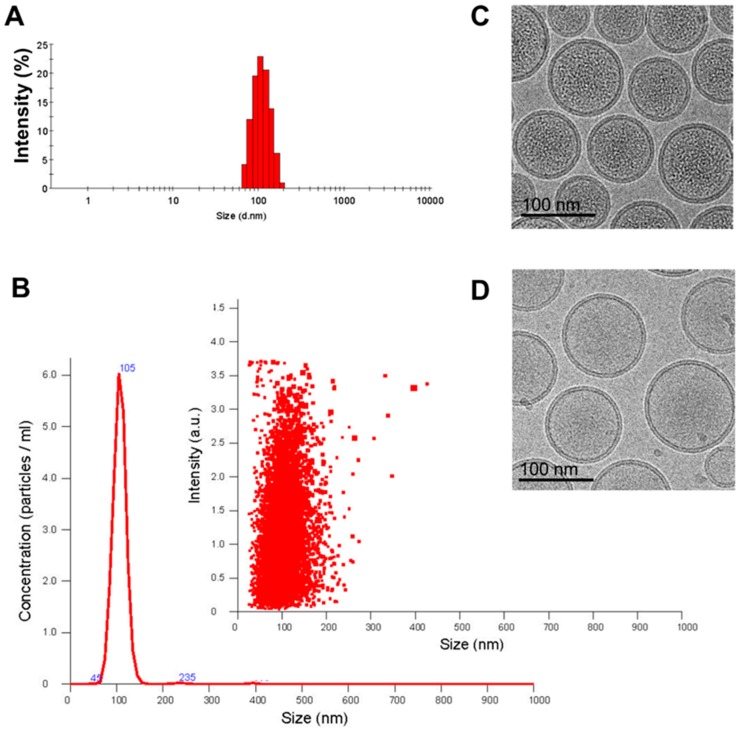
Figure 1.
Characterization of Lipo-IRI (irinotecan) formulation. The size of Lipo-IRI was estimated using (A) dynamic light scattering (DLS) and (B) nanoparticle tracking analysis (NTA). The size distribution and particle concentration were calculated as intensity and particles/ml, respectively. The cryo-transmission electron microscopy (TEM) imaging was performed on a drug-loaded 1,2-Distearoyl-sn-glycero-phosphocholine (DSPC)/Cholesterol formulation either (C) with or (D) without irinotecan (IRI). (10 μmol/mL phospholipid; scale bar represents 100 nm).