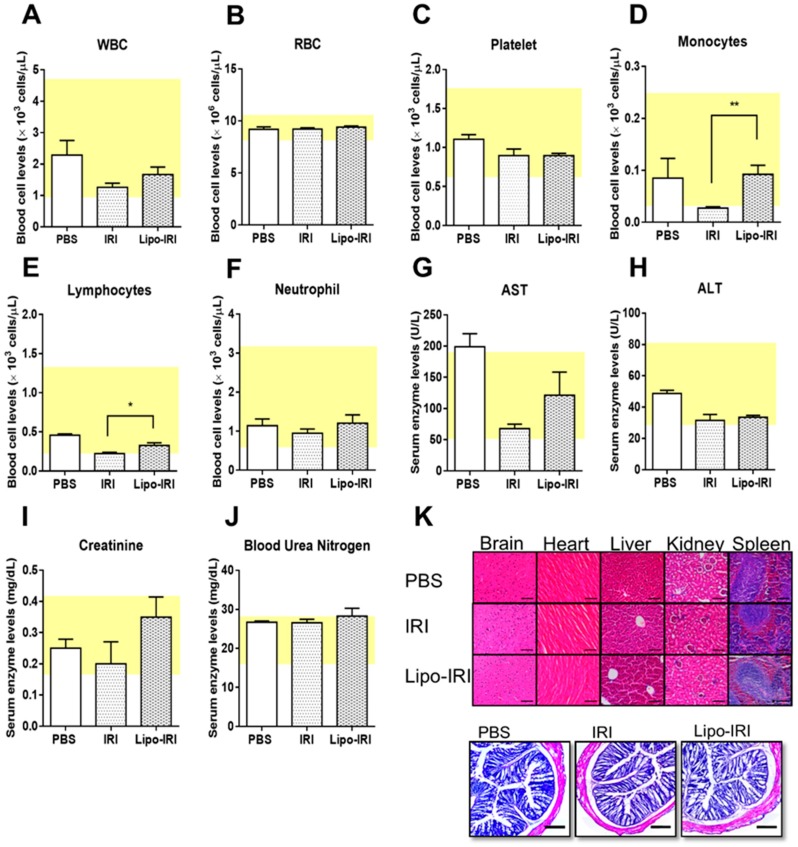
Figure 5.
Evaluation of side effects in hematological and histological study. The mice were treated with either IRI or Lipo-IRI at 5 mg/kg twice weekly. The plasma and serum were harvested and analyzed. The hematology for (A) white blood cell (WBC), (B) red blood cell (RBC), (C) platelet, (D) monocyte, (E) lymphocyte, and (F) neutrophil are shown. The (G) aspartate aminotransferase (AST), (H) alanine aminotransferase (ALT), (I) creatinine, and (J) blood urea nitrogen levels were measured to assess the hepatotoxicity and nephrotoxicity. The data are presented as the mean ± SEM (n = 3). The yellow box indicates the normal range. Compared to IRI, * p < 0.05. (K) The major organs were harvested for paraffin embedding, and the colon was frozen in optimal cutting temperature (OCT) compound after the end of the treatment. Tissue sections were stained with H&E for a histological analysis (Scale bars, 200 μm).