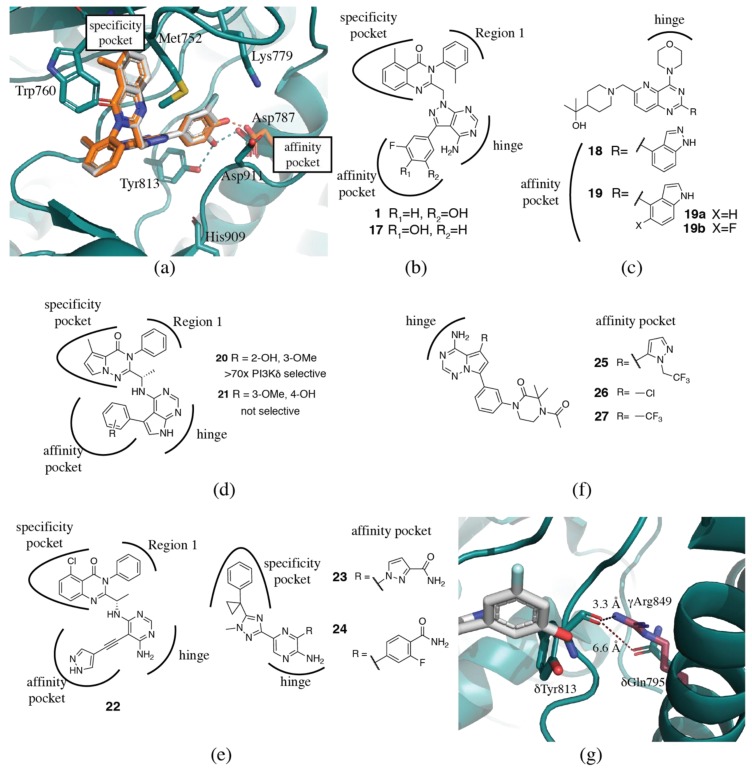
Figure 4.
Modifications in the affinity pocket. (a) Impact of subtle differences in the affinity pocket. meta-substituted fluorophenol 1 (white, PDB ID 2WXG) can form hydrogen bonds with δAsp787 and δTyr813 (indicated with teal dashed lines), while ortho-substituted fluorophenol 17 (orange, PDB ID 2WXH) can only form hydrogen bonds with δAsp787 (indicated with orange dashed lines) (b) Structures of 1 and 17 (c) Structures of inhibitors with indole and indazole affinity pocket binding motifs (d) Structures of inhibitors 20–21 (e) Structures of inhibitors 22–24 (f) Structures of inhibitors 25–27 (g) Rationalizing differences in affinity pocket selectivity. γArg849 forms a hydrogen bond with the backbone of γTyr867 (equivalent to δTyr813), restricting movement in the affinity pocket, while the side-chain of δGln795 is too short to make the same interaction. Distances are marked with dashed lines. The fluorophenol moiety of 1 bound in the affinity pocket is shown for reference.