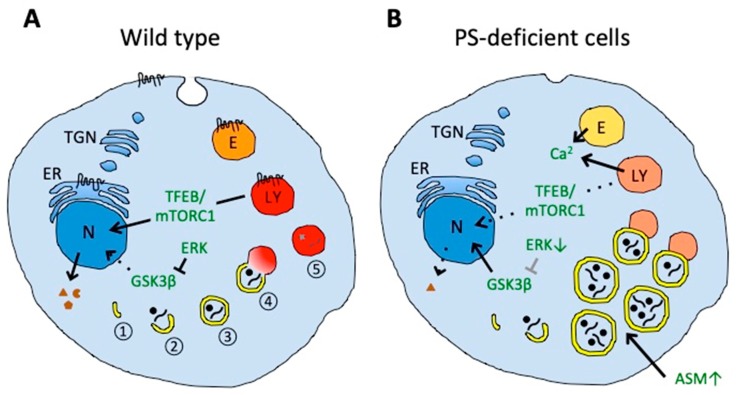
Figure 2.
Impaired autophagy and lysosomal degradation in PS-deficient cells. (A) In wild-type cells, autophagy includes nucleation (1) and elongation of phagophores (2), autophagosome formation by phagophore maturation (3), autolysosome formation by the fusion of autophagosomes and lysosomes (4), and final degradation of the contents (5). (B) In PS-deficient cells, enlarged autophagic vacuoles accumulate and contain undigested engulfed material. The accumulation of autophagic vacuoles could result from the disturbed fusion of autophagosomes with lysosomes, probably caused by impaired lysosomal acidification (illustrated in a pale red color). Aberrant acidification could also affect calcium homeostasis in endolysosomal vesicles that could contribute to impaired vesicle fusion. PS deficiency can also impair amino acid sensing by mTORC1 on lysosomes and decrease activation and nuclear translocation of transcription factor EB (TFEB), thereby decreasing expression of proteins mediating biogenesis of lysosomal and autophagic vesicles. Decreased translocation of glycogen synthase kinase 3β (GSK3β) from the cytosol to the nucleus could also decrease the activation of TFEB. Increased acid sphingomyelinase (ASM) in PS-deficient cells can induce the accumulation of autophagic vacuoles. Decreased endocytosis in PS-deficient cells could also affect membrane protein and lipid homeostasis. Presenilin can be localized in the endoplasmic reticulum (ER), plasma membrane, endosomes (E), and lysosomes (LY). N, nucleus; TGN, trans-Golgi network.