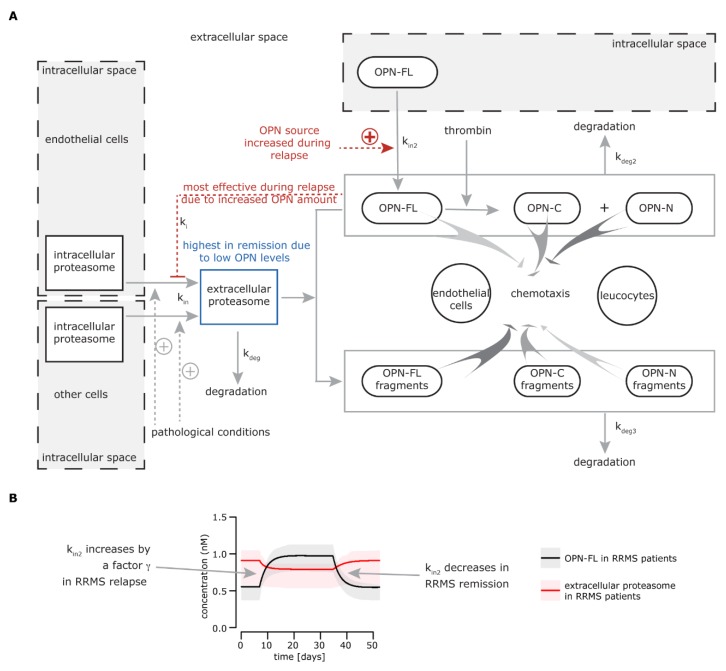
Figure 1.
Extracellular osteopontin-(OPN) proteasome circuit model. (A) The biological model behind the mathematical model: intracellular proteasomes are released by endothelial cells and other cell types (not identified) into the extracellular space (kin), where they can be degraded (kdeg). This physiological phenomenon could be enhanced in relapsing-remitting multiple sclerosis (RRMS) and other chronic diseases or upon traumatic injury, thereby leading to a chronically larger amount of proteasomes in the extracellular space. The release of proteasomes by endothelial cells is inhibited by OPNs (ki) and possibly other unknown factors, whereas other cells are insensitive to OPN-mediated inhibition of proteasome release. ki is prominent during the RRMS relapse when OPN levels are higher, leading to a decrease of proteasome release in the extracellular space. The pathological levels of extracellular proteasomes are restored in the remission when the OPN concentration is diminished. In the extracellular space, full length secreted OPN (OPN-FL) can be fragmented in OPN-C-terminal (OPN-C) and OPN-N-terminal (OPN-N) by thrombin or processed by other proteases. Extracellular proteasomes can cleave OPN-N (kdeg3), thereby partially compromising its chemotactic activity. The peptide fragments generated by proteasomes via processing OPN-FL and OPN-C (kdeg3) have an enhanced chemotactic activity compared to the parental proteins, thereby promoting the chemotaxis of different cell types such as endothelial cells, lymphocytes, and monocytes [25]. (B) The simulation of OPN-FL and extracellular (standard) proteasome concentrations over time for RRMS patients. The time window is arbitrarily set. The behavior of OPN-FL during relapse is given, and the behavior of extracellular standard proteasomes is a result of the modeled system. Here, we assume that only OPN-FL is present in the extracellular space. The secretion of OPN-FL from cells in the extracellular (kin2) increases during (or right before) the relapse in serum of RRMS patients by a γ factor. kin2 decreases at the end of the relapse.