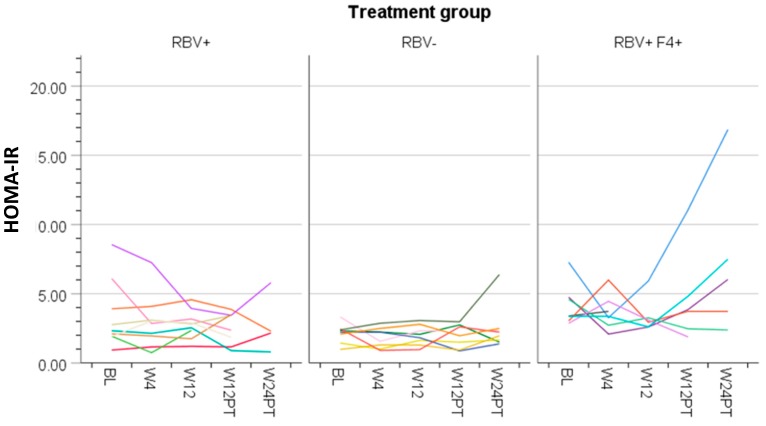
Figure 1.
Homeostatic Model Assessment—Insulin Resistance (HOMA-IR) at each study time point (baseline, Week 4 of treatment, end of treatment at Week 12, 12 weeks after treatment completion, and 24 weeks after treatment completion) for each participant by treatment group (RBV+ = ribavirin containing hepatitis C (HCV) treatment in participants without cirrhosis, RBV− = ribavirin free HCV treatment in participants without cirrhosis, RBV+F4+ = ribavirin containing HCV treatment in participants with cirrhosis).