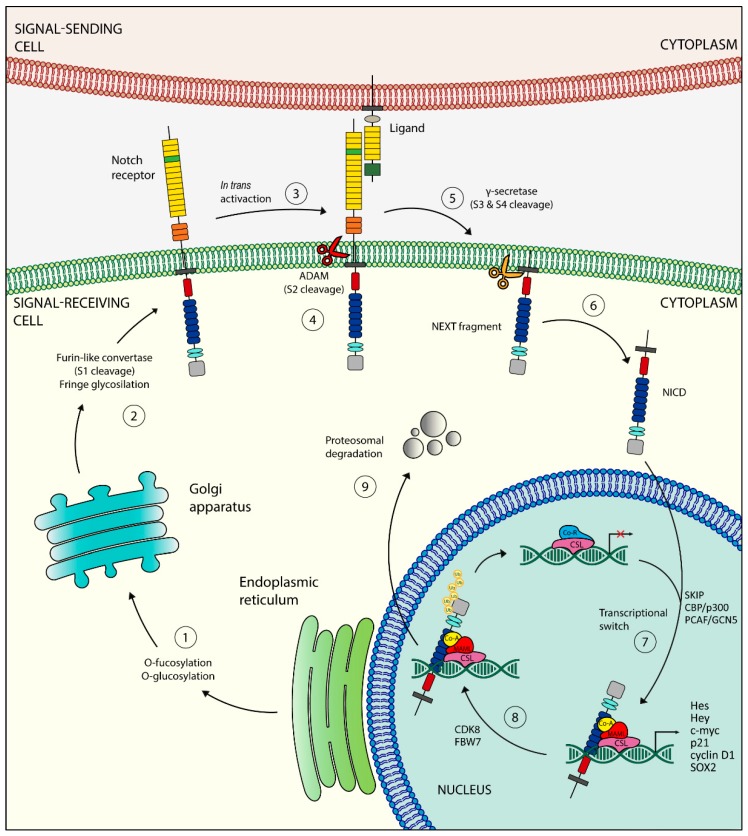
Figure 2.
Schematic representation of the Notch signaling pathway. Once synthesized in the endoplasmic reticulum (①), the inactive single peptide precursor moves to the Golgi where it is cleaved by a furin-like convertase (S1 cleavage) (②) and translocates into the cell membrane. The binding with a Notch ligand (③) induces the second cleavage (S2) by a member of the disintegrin and metalloproteinases (ADAM) family (④), resulting in the formation of a membrane-tethered Notch truncated (NEXT) fragment, which is further processed in two sites (S3 and S4) by a presenilin-dependent γ-secretase complex (⑤), generating the Notch intracellular domain (NICD), the active form of the Notch receptor (⑥). The NICD can now enter into the nucleus, where it exerts its transcriptional activity (⑦). The ubiquitination of the NICD (⑧) leads to its proteasome degradation (⑨).