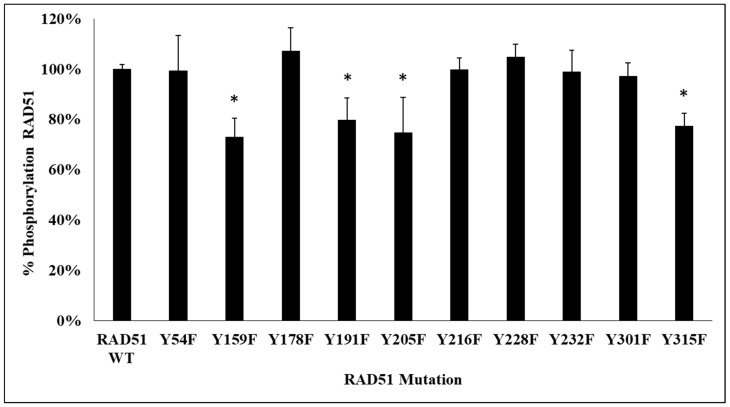
Figure 2.
Decrease in RAD51 phosphorylation level in 4 RAD51 mutants. RAD51 WT protein and all non-phosphorylatable mutants of RAD51 (10 µM) were purified and incubated in the presence of kinase-active c-MET (0.1 µM) and ATP (2 mM). Then, the proteins were separated by SDS-PAGE 12% gel and analyzed by western blot using anti-RAD51 and anti-phosphotyrosine antibodies. The signals were quantified with the Odyssey scanner and compared with the signal from RAD51 WT. The graph represents the phosphorylation percentage of each mutant compared to the wild protein and obtained from the quantification of blots. Each experiment was conducted three times independently (n = 3; error bars: s.d.; * p < 0.05).