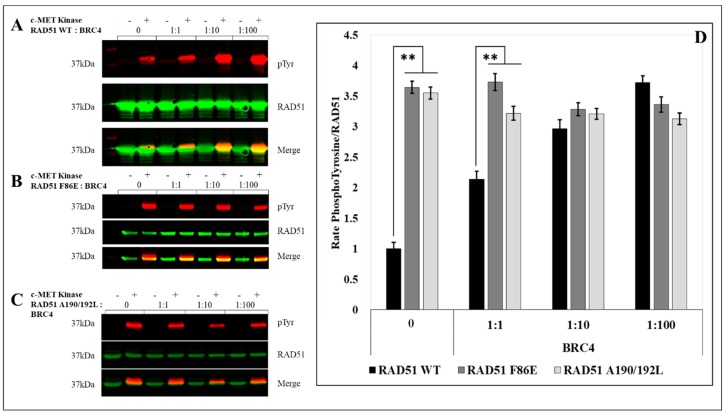
Figure 5.
c-MET-mediated RAD51 phosphorylation is related to its oligomeric state. (A) The RAD51 WT protein (10 µM) and all non-polymerizable mutants of RAD51: (B) F86E and (C) A190/192L were purified and incubated with ATP (2 mM), with or without kinase-active c-MET (0.1 µM), and an increasing concentration of BRC4 peptide. Then the proteins were separated by SDS-PAGE 12% gel and analyzed by western blot using anti-RAD51 (green) and anti-phosphotyrosine (red) antibodies. The signals were quantified by Odyssey scanner and compared with the signal from RAD51 WT. (D) The graph represents the phosphorylation rate of each mutant compared to the wild protein following the quantification of blots from three independent experiments (n = 3; errors bars: s.d.; ** p < 0.01).