Fig. 2.
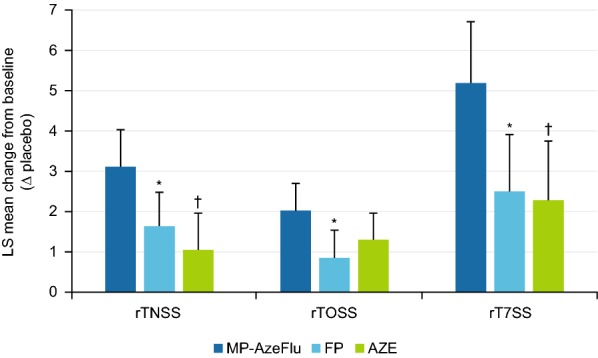
Change from baseline in reflective total nasal symptom score (rTNSS), reflective total ocular symptom score (rTOSS), and reflective total of seven symptom scores (rT7SS) following 14 days’ treatment with MP-AzeFlu (n = 153), fluticasone propionate (FP; n = 151), or azelastine hydrochloride (AZE; n = 152). LS least squares, MP-AzeFlu a novel formulation of an intranasal antihistamine, azelastine, and an intranasal corticosteroid, fluticasone propionate, in a single spray. The precision of these estimates is indicated by the upper bounds of the respective 95% confidence intervals. *p ≤ 0.0031 versus MP-AzeFlu. †p ≤ 0.0004 versus MP-AzeFlu
(Modified from Meltzer et al. [118])
