Fig. 4.
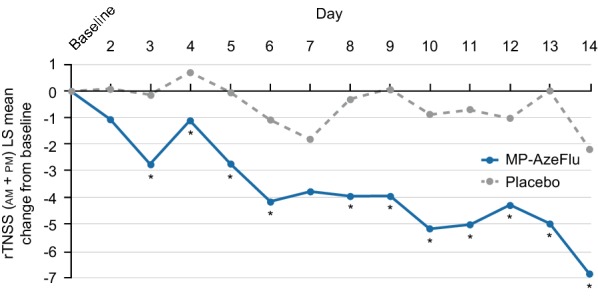
Least squares (LS) mean change from baseline in reflective total nasal symptom score (rTNSS; am + pm) per day when most children (n = 82; > 90%) rated their own symptoms following treatment with MP-AzeFlu or placebo, both one spray per nostril twice daily, for 14 days in children aged 6–11 years with moderate-severe SAR. MP-AzeFlu a novel formulation of an intranasal antihistamine, azelastine hydrochloride, and an intranasal corticosteroid, fluticasone propionate, in a single spray, SAR seasonal allergic rhinitis. *p ≤ 0.040 versus placebo
(Reprinted with permission from Berger et al. [130])
