Abstract
Primary clear cell carcinoma of the liver (PCCCL) is a rare and special type of primary hepatocellular carcinoma. However, treatment methods for multiple metastatic PCCCL are lacking. Here, we report the case of a 55-year-old male PCCCL patient with multiple metastatic lesions who was clinically cured by sunitinib-based systematic treatment. This patient was diagnosed with PCCCL in Liver Segment 7, Child–Pugh A liver function, Stage A in November 16, 2009, and received radical excision of the cancer immediately. His disease recurred with multiple metastatic lesions in the liver and other parts of the body, including the retroperitoneal lymph nodes, lung and bilateral adrenal nodules in June 29, 2012. The biopsy results showed that the lung mass was lung metastasis of PCCCL. With Child–Pugh A liver function, Stage C of PCCCL was diagnosed. Sunitinib (37.5 mg, oral, once a day [qd]) in combination with Chinese herbal medicine (CHM) was given. The tumor size steadily reduced, and the lesions were no longer obvious in May 21, 2014. The patient had multiple metastases and is in complete response (CR) state until now. He is considered as clinically cured. From the initial diagnosis of PCCCL, the survival period reached 8 years.
Keywords: hepatocellular carcinoma, primary clear cell carcinoma of the liver, sunitinib, anti-angiogenesis therapy, Chinese herbal medicine
Introduction
Primary clear cell carcinoma of the liver (PCCCL) is a rare and special type of primary hepatocellular carcinoma with incidence rates of 0.4%–37%.1–9 In metastatic patients unable to receive surgery, the efficacy of chemotherapy and radiotherapy is very limited,3 and to date, other reliable treatment methods are lacking. Here, we report a PCCCL patient with multiple metastatic lesions who was clinically cured by sunitinib-based systematic treatment. This study may provide a reference for treating patients with multiple metastases who cannot undergo surgery.
Case presentation
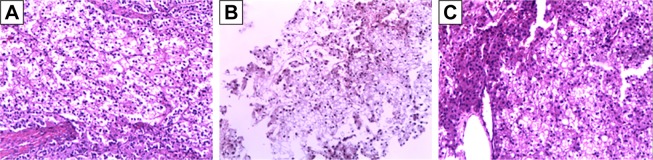
A 55-year-old Asian man, who had no history of smoking, drinking, and hepatitis but had hypertension for 10 years, was diagnosed with PCCCL in Liver Segment 7, Child– Pugh A liver function, Stage A according to the Barcelona Clinic Liver Cancer staging system in November 16, 2009, during a physical examination. He received radical excision of cancer in December 3, 2009. Histology report showed the following: the maximum diameter of the tumor was 5.5 cm, and no vascular or membrane infiltration was seen. The results of H&E staining are shown in Figure 1A.
Figure 1.
Pathological characteristics demonstrated by H&E staining of the patient (100×).
Notes: A large number of transparent cells with diffuse distribution were observed in the primary liver tumor, lung metastatic lesion, and adrenal metastatic lesion. (A) Histopathology of primary liver tumor in December 3, 2009. (B) Histopathology of lung metastatic lesion in July 20, 2012. (C) Histopathology of adrenal metastatic lesion in October 13, 2017.
The patient took Chinese herbal medicine (CHM) orally after the surgery in December 3, 2009, and received regular follow-up until June 29, 2012. No side effects were noted. The treatment principle of CHM is to invigorate the spleen and remove liver stasis. The specific prescription and its dose are the same as what we have reported previously for hepatocellular carcinoma.10 In September 17, 2010, a nodular shadow with a 0.5 cm diameter on the medial branch of the left adrenal gland was tested by enhanced computed tomography (CT), and it was considered most likely to be an adenoma, probably a metastasis. No change in the size of the adrenal gland nodule was observed in the following months. Multiple nodules with a maximum diameter of approximately 1.7 cm, clear boundaries, and moderate enhancement in the right adrenal gland were detected by CT in July 1, 2011, and no change in size was observed in the following months.
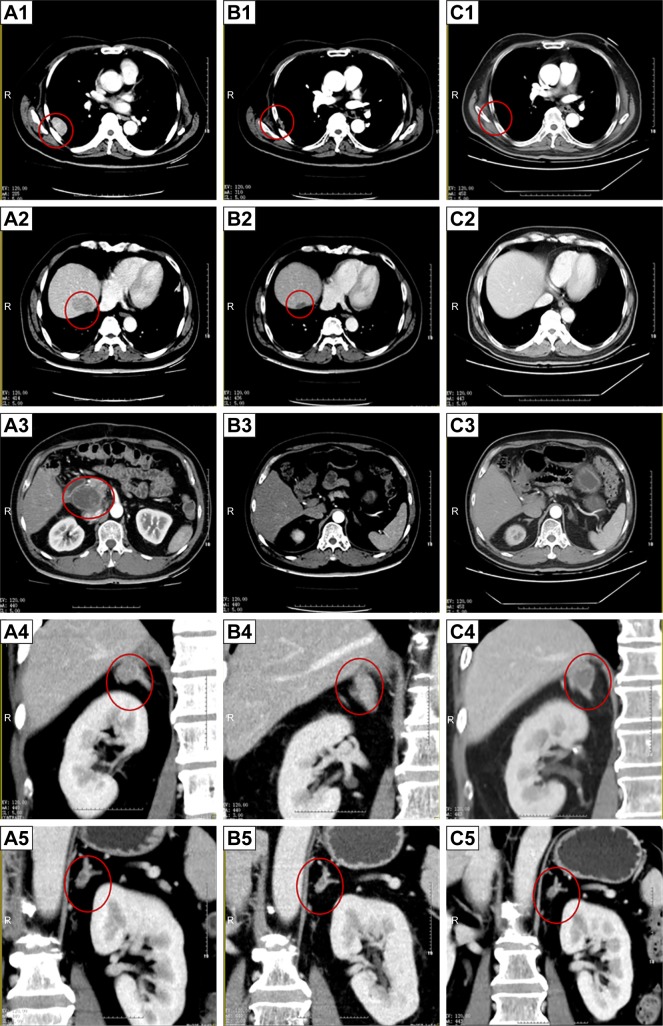
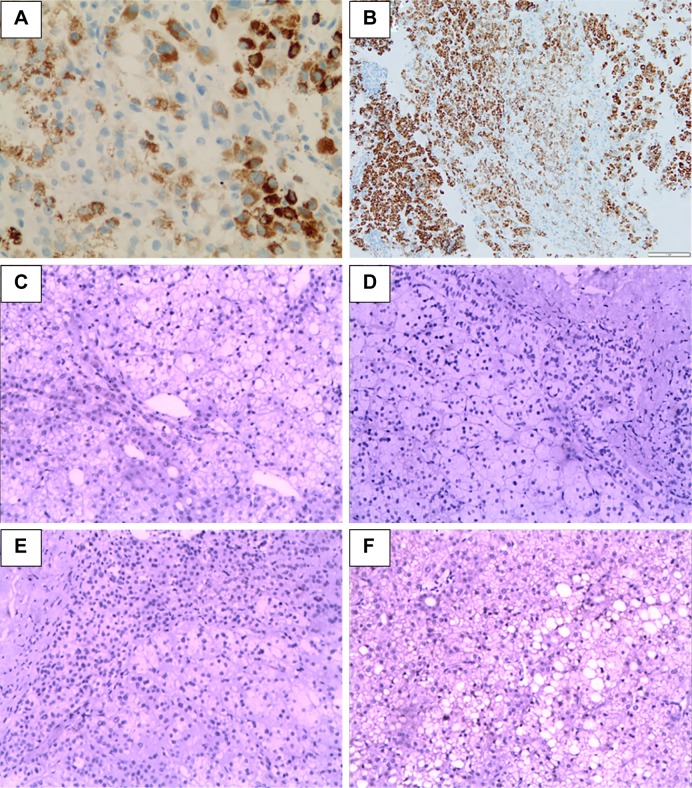
According to the result of a thorough examination in June 29, 2012, the patient’s disease has recurred with multiple metastatic lesions in the liver and other parts of the body, including the retroperitoneal lymph nodes, lung and bilateral adrenal nodules based on the CT scan (Figure 2A). The blood test results showed that the concentration of serum CA12-5 was 57.86 U/mL higher than the normal value, and the serum alpha fetoprotein concentration was within normal range. Biopsy of the lung mass was carried out. Based on the pathological characteristics demonstrated by H&E (Figure 1B) and immunohistochemical staining in the lung mass; the positive expression of hepatocytes and CK (Figure 3A); and the negative expression of vimentin, the lung mass was lung metastasis derived from the primary liver tumor. With Child–Pugh A liver function, Stage C of PCCCL was diagnosed. Cryotherapy for lung metastasis was performed at the same time as biopsy. The patient refused to receive sorafenib due to heavy economic burden. Considering that sunitinib might have an inhibition effect on PCCCL, which is effective for renal clear cell carcinoma, sunitinib (37.5 mg, oral administration, once a day [qd]) in combination with CHM (the same as above) was given from July 13, 2012, until his recovery from cryotherapy. The patient experienced skin peeling and diarrhea, since day 15 of sunitinib administration. To improve the side effects of sunitinib, we changed CHM treatment into Invigorating the Spleen and Nourishing Kidney Yin, Eliminating Dampness, and Removing the Stasis. The formula consists of 15 g Dang Shen (codonopsis root), 15 g Bai Zhu (ovate atractylodes rhizome), 25 g Fu Ling (poria), 30 g Xian He Cao (hairy vein agrimonia), 20 g Nv Zhen Zi (privet fruit), 20 g Mo Han Lian (yerbadetajo herb), 20 g Sheng Di (rehmannia root), 15 g Dan Pi (tree peony bark), 30 g Dan Shen (danshen root), 15 g Er Zhu (curcumae rhizome), 10 g Ku Shen (light yellow sophora root), 15 g Ze Xie (alisma rhizome),15 g Huo Xiang (agastache), and 10 g Dang Gui (Chinese angelica). The side effects of sunitinib were completely cured after 3 months of CHM oral administration. Regular follow-up tests were performed every 3 months. No abnormal blood cell count, liver function damage, or kidney function damage was noted. The serum concentration of CA12-5 was within the normal range, since November 3, 2013. The tumor size steadily reduced, and the lesions were no longer obvious in May 21, 2014 (Figure 2B).
Figure 2.
CT images of the patient.
Notes: (A1–A5) Images at the start of sunitinib administration (June 29, 2012). Multiple metastatic lesions in the liver (A2) and other parts of the body, including the retroperitoneal lymph nodes (A3), lung (A1), and bilateral adrenal nodules (A4 and A5), were observed. (B1–B5) Two years after the start of sunitinib administration (May 21, 2014). Marked decrease in the size of all metastatic lesions was observed. (C1–C5) Images at 4.75 years after the start of sunitinib administration (March 2, 2017). All intrahepatic tumors had shrunk, no enhancement of tumor staining was observed in the arterial phase, and the lung metastasis had disappeared.
Abbreviation: CT, computed tomography.
Figure 3.
Immunohistochemical staining of hepatocytes, PDGFR, and VEGFR in the primary tumor and metastatic lesion.
Notes: (A) High expression of hepatocytes in the lung metastatic lesion in July 20, 2012 (200×). (B) High expression of hepatocytes in the adrenal metastatic lesion in October 13, 2017 (100×). (C and E) Negative expression of PDGFR and VEGFR in the primary liver tumor in December 3, 2009 (100×). (D and F) Negative expression of PDGFR and VEGFR in the adrenal metastatic lesion in October 13, 2017 (100×).
Abbreviations: PDGFR, platelet-derived growth factor receptor; VEGFR, vascular endothelial growth factor receptor.
A right adrenal nodule with a maximum diameter of 1.3 cm reappeared at the original site in November 25, 2014. Its maximum diameter increased to 3.5 cm in December 4, 2015. No obvious lesions were noted in other locations. Sunitinib was continuously taken, but CHM was discontinued due to patient’s refusal to take the medicine daily in December 11, 2015. Then, the patient discontinued sunitinib due to size reduction of the adrenal gland nodule (2.3 cm maximum diameter) in August 5, 2016.
The maximum diameter of the right adrenal mass decreased to 1.5 cm in March 2, 2017 (Figure 2C) and slightly increased to 2.1 cm in September 20, 2017. Because of the enlargement, right adrenal mass resection was performed under laparoscopy in October 13, 2017. Histopathology showed that the mass was an adrenal metastatic PCCCL based on its pathological characteristics demonstrated by H&E (Figure 1C) and immunohistochemical staining, which exhibited the high expression of hepatocytes and glypican (Figure 3B).
The patient had multiple metastases and is in complete response (CR) state until now. He is considered as clinically cured. From the initial diagnosis of PCCCL, the survival period has reached 8 years. To determine the mechanism behind the curative effect of sunitinib, we evaluated the expression of PDGFR and VEGFR in the primary liver lesions (December 3, 2009) and adrenal metastases (October 13, 2017). We found that PDGFR and VEGFR were not expressed in these tissues (Figure 3C–F).
Discussion
This patient has survived 5 years since the discovery of multiple liver nodules and multiple metastases. What is more important is that the multiple metastatic lesions disappeared after sunitinib-based systematic treatment. By searching in the PubMed and Web of Science databases, we found that this is the first case of PCCCL treatment using sunitinib combined with CHM.
PCCCL is characterized by a large number of transparent cells with diffuse distribution. To date, surgical resection is considered to be the best treatment for early stage clear cell carcinoma of the liver. Most patients have good curative effect and a good long-term survival rate.3,4,7,9,11 However, for the few case reports of unresectable PCCCL patients, this disease is not sensitive to chemotherapy and radiotherapy, and the survival time is unsatisfactory.12–14 Thus, no feasible treatment for unresectable patients exists to date.
Sunitinib was assumed to play a key role behind the therapeutic effect in this patient. It is an oral multitarget kinase inhibitor, which has anti-tumor and anti-angiogenesis effects. Sunitinib can inhibit multiple kinases, including VEGFR-1, 2, and 3; PDGFR-α; PDGFR-β; stem cell factor receptor; FMS-like tyrosine kinase 3; colony-stimulating factor receptor 1; and rearranged during transfection. These pathways are related to tumor growth, angiogenesis, and metastasis. In animal experiments, sunitinib has stronger anti-VEGFR activity than sorafenib.15 Sunitinib has been approved for use in advanced renal clear cell carcinoma.16 In a clinical study of hepatocellular carcinoma, sunitinib prolonged the survival of Asian and hepatitis B patients similar to sorafenib, but it was not recommended due to its higher rate of severe side effects.17 Based on the efficacy of sunitinib for renal clear cell carcinoma and hepatocellular carcinoma, we speculated that it may be effective in our patient. After receiving sunitinib, the patient’s liver, lung, retroperitoneal, and adrenal lesions disappeared; however, the right adrenal gland lesion recurred.
The expression of VEGFR and PGDFR in the primary tumor and metastatic lesions was negative. The expression of VEGFR and PDGFR in lung metastatic lesions was not evaluated because of sample limitations. Consequently, we could not determine the mechanism behind the therapeutic effect of sunitinib. We formulated one hypothesis for this situation: sunitinib might exhibit anti-tumor effects through other targets because PCCCL has a low rate of vascular invasion.4 Despite the unclear mechanism of the therapeutic effect, some patients with special type of HCC and PCCCL could benefit from sunitinib.
CHM might have also played an important role in the treatment of the patient. The usage of CHM after the radical surgery might have reduced the postoperative recurrence and metastasis of the disease.18,19 It could have improved the effect of sunitinib,10 because CHM treatment could partly change the immune and vascular microenvironments of tumor growth, which might increase patients’ chances of benefit.20 Moreover, CHM might have improved the side effects of sunitinib as determined by the improvement of skin peeling and diarrhea after 3 months of CHM oral administration.
Sunitinib is not recommended for the treatment of liver cancer due to its severe side effects according to a previous study.21 In the present study, sunitinib combined with CHM might be an optional treatment method for liver cancer patients given the improved side effects and efficacy.
Because this is only a case study, the evidence level of this treatment approach is very limited. However, in the current setting where there are not much treatment methods available and high-level evidence-based research is difficult to achieve, our study is a very worthwhile approach. Of course, it is worthy of recommendation if we can identify the degree of angiogenesis by immunohistochemistry before treatment or whether gene sequencing can determine the associated VHL gene and other genes for individualized precise treatment.22
Conclusion
A patient with metastatic PCCCL satisfactorily responded to sunitinib- and-CHM-based systematic treatment. However, more studies on this potential treatment method are needed to confirm our findings.
Ethics approval and consent to participate
This study was conducted in accordance with the standards of the Declaration of Helsinki for medical research involving human subjects. The patient provided written informed consent for the publication of this report and the accompanying images, and the study protocol was approved by the clinical research ethical review board at Guangzhou University of Chinese Medicine, First Affiliated Hospital. Institutional approval was not required to publish this manuscript.
Acknowledgments
The authors thank Dr Huangfang Zheng from Singapore, and Dr Hezheng Lai and Prof Xiaoshu Zhu from Australia for helping to polish the language. We greatly appreciate the patient and his family for their kind cooperation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Liu QY, Li HG, Gao M, et al. Primary clear cell carcinoma in the liver: CT and MRI findings. World J Gastroenterol. 2011;17(7):946–952. doi: 10.3748/wjg.v17.i7.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lao XM, Zhang YQ, Jin X, et al. Primary clear cell carcinoma of liver–clinicopathologic features and surgical results of 18 cases. Hepatogastroenterology. 2006;53(67):128–132. [PubMed] [Google Scholar]
- 3.Ji SP, Li Q, Dong H. Therapy and prognostic features of primary clear cell carcinoma of the liver. World J Gastroenterol. 2010;16(6):764–769. doi: 10.3748/wjg.v16.i6.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, Ma W, Li H, et al. Clinicopathological and prognostic features of primary clear cell carcinoma of the liver. Hepatol Res. 2010;38(3):291–299. doi: 10.1111/j.1872-034X.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- 5.Kashala LO, Conne B, Kalengayi MM, Kapanci Y, Frei PC, Lambert PH. Histopathologic features of hepatocellular carcinoma in Zaire. Cancer. 1990;65(1):130–134. doi: 10.1002/1097-0142(19900101)65:1<130::aid-cncr2820650126>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Xu W, Ge P, Liao W, et al. Edmondson grade predicts survival of patients with primary clear cell carcinoma of liver after curative resection: a retrospective study with long-term follow-up. Asia Pac J Clin Oncol. 2017;13(5):e312–e320. doi: 10.1111/ajco.12494. [DOI] [PubMed] [Google Scholar]
- 7.Lai CL, Wu PC, Lam KC, Todd D. Histologic prognostic indicators in hepatocellular carcinoma. Cancer. 1979;44(5):1677–1683. doi: 10.1002/1097-0142(197911)44:5<1677::aid-cncr2820440522>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Emile JF, Lemoine A, Azoulay D, Debuire B, Bismuth H, Reynès M. Histological, genomic and clinical heterogeneity of clear cell hepatocellular carcinoma. Histopathology. 2010;38(3):225–231. doi: 10.1046/j.1365-2559.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang SH, Watanabe J, Nakashima O, Kojiro M. Clinicopathologic study on clear cell hepatocellular carcinoma. Pathol Int. 1996;46(7):503–509. doi: 10.1111/j.1440-1827.1996.tb03645.x. [DOI] [PubMed] [Google Scholar]
- 10.Sun L, Fahey P, Zhu X, et al. A cohort study to examine the use of Chinese herbal medicine in combination with conventional therapies for patients with hepatocellular carcinoma in China. Integr Cancer Ther. 2018;17(3):902–911. doi: 10.1177/1534735418775819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T, Fan J, Qin LX, et al. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011;18(7):1955–1963. doi: 10.1245/s10434-010-1540-z. [DOI] [PubMed] [Google Scholar]
- 12.Shah S, Gupta S, Shet T, Maheshwari A, Wuntkal R, Mohandas KM. Metastatic clear cell variant of hepatocellular carcinoma with an occult hepatic primary. Hepatobiliary Pancreat Dis Int. 2005;4(2):306–307. [PubMed] [Google Scholar]
- 13.Maharajan K, Hey HWD, Tham I, et al. Solitary vertebral metastasis of primary clear cell carcinoma of the liver: a case report and review of literature. J Spine Surg. 2017;3(2):287–293. doi: 10.21037/jss.2017.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong J, He D, Hu W, Liu X. Retroperitoneal and intrahepatic metastasis from primary clear cell carcinoma of the liver: A case report and review of the literature. Medicine (Baltimore) 2017;96(12):e6452. doi: 10.1097/MD.0000000000006452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu AX, Duda DG, Sahani DV, Jain RK. Development of sunitinib in hepatocellular carcinoma: rationale, early clinical experience, and correlative studies. Cancer J. 2009;15(4):263–268. doi: 10.1097/PPO.0b013e3181af5e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JJ, Vaziri SA, Rini BI, et al. Association of VEGF and VEGFR2 single nucleotide polymorphisms with hypertension and clinical outcome in metastatic clear cell renal cell carcinoma patients treated with sunitinib. Cancer. 2012;118(7):1946–1954. doi: 10.1002/cncr.26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 18.Zhong C, Li HD, Liu DY, et al. Clinical study of hepatectomy combined with Jianpi Huayu therapy for hepatocellular carcinoma. Asian Pac J Cancer Prev. 2014;15(14):5951–5957. doi: 10.7314/apjcp.2014.15.14.5951. [DOI] [PubMed] [Google Scholar]
- 19.Zhong C, Zhang YF, Huang JH, et al. The Chinese medicine, Jianpi Huayu decoction, inhibits the epithelial mesenchymal transition via the regulation of the Smad3/Smad7 cascade. Am J Transl Res. 2017;9(6):2694–2711. [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Chen LY, Wang P, et al. Tumor microenvironment varies under different TCM ZHENG models and correlates with treatment response to herbal medicine. Evid Based Complement Alternat Med. 2012;2012:1–10. doi: 10.1155/2012/635702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 22.Shell J, Patel D, Powers A, et al. Somatic VHL mutation in a patient with MEN1-associated metastatic pancreatic neuroendocrine tumor responding to sunitinib treatment: a case report. J Endocr Soc. 2017;1(9):1124–1134. doi: 10.1210/js.2017-00156. [DOI] [PMC free article] [PubMed] [Google Scholar]