Abstract
Kaempferol, one of the phytoestrogens, is found in berries and Brassica and Allium species and is known to have antioxidative and anti-inflammatory properties. In the present study, we examined the molecular mechanisms underlying the anti-inflammation effect of kaempferol in an aged animal model. To examine the effect of kaempferol in aged Sprague-Dawley rats, kaempferol was fed at 2 or 4 mg/kg/day for 10 days. The data show that kaempferol exhibited the ability to maintain redox balance. Kaempferol suppressed nuclear factor-κB (NF-κB) activation and expression of its target genes cy-clooxygenase-2, inducible nitric oxide synthase, monocyte chemoattractant protein-1, and regulated upon activation, and normal T-cell expressed and secreted in aged rat kidney and in tert-butylhydroperoxide-induced YPEN-1 cells. Furthermore, kaempferol suppressed the increase of the pro-inflammatory NF-κB cascade through modulation of nuclear factor-inducing kinase (NIK)/IκB kinase (IKK) and mitogen-activated protein kinases (MAPKs) in aged rat kidney. Based on these results, we concluded that anti-oxidative kaempferol suppressed the activation of inflammatory NF-κB transcription factor through NIK/IKK and MAPKs in aged rat kidney.
Key Words: aging, anti-inflammation, kaempferol, mitogen-activated protein kinases, nuclear factor-κB, nuclear factor-inducing kinase/IκB kinase
Introduction
Phytoestrogens are plant-derived, nonsteroidal compounds that bind to estrogen receptors and produce estrogen-like activity.1 They have attracted much attention among the public and medical communities because of their potential beneficial role in the prevention and treatment of cardiovascular diseases, osteoporosis, diabetes, and obesity.2,3 The anti-inflammatory action of kaempferol, a phytoestrogen, is reported to inhibit the gene expression of lipopolysaccharide-induced interleukin 1β and tumor necrosis factor-α (TNF-α) in J774.2 macrophages.4 Also, kaempferol has been shown to efficiently modulate redox status, for which balance may play a role in the regulation of inflammatory mediators such as TNF-α and cyclooxygenase-2 (COX-2).5 Recent studies have demonstrated that kaempferol's anti-inflammatory effects down-regulate the nuclear factor-κB (NF-κB) pathway in a cell culture system.6,7
However, the effectiveness of kaempferol as a reduction-oxidation (redox) modulator on pro-inflammatory NF-κB activation and its signal pathway in aged rat kidney has not been studied.
Aging is characteristically described as time-dependent functional declines that lead to inability to withstand external and internal changes, resulting in eventual homeostatic failure. Currently, the best mechanistic elucidations of aging are offered by the oxidative stress hypothesis, which proposes the cellular redox imbalance is the underlying cause of aging.8,9 One of the major consequences of redox disruption is the well-known age-related chronic inflammatory process.10,11 Recent reviews published from our laboratory elaborated on the interrelation between oxidative stress and inflammations during aging and highlighted redox-sensitive NF-κB as playing a key role in the pro-inflammatory state of aged organisms.11,12
NF-κB is one of the principal inducible transcription factors shown to respond directly to oxidative stress.13,14 Under normal physiological conditions, NF-κB exists in the cytoplasm as an inactive form complexed by its inhibitory subunit, IκBα. Oxidative stress activates nuclear factor-inducing kinase (NIK)/IκB kinase (IKK) and mitogen-activated protein kinases (MAPKs).15–18 NIK/IKK and MAPK pathways then translocate activated NF-κB to the nucleus and permit the binding of NF-κB to regulatory elements in DNA enhancers and promote subsequent to gene expression.19,20
For our present study, we chose to examine kidney tissue and YPEN-1 cells. Kidney consists of endothelial and epithelial cells, and it is metabolically active, sensitive to many age-related changes in blood vessel tone determined by the balance between vasoconstrictors and vasodilators. Moreover, endothelial cells are easily affected by age-related changes.21 Therefore, we chose kidney and the endothelial cell type YPEN-1 cell line for the study. Recently, our laboratory presented evidence that rat kidney tissue and the YPEN-1 cell line are used to increase NF-κB activation during aging.22
In the present study, we examined the efficacy of the molecular modulation mechanism of kaempferol's anti-oxidative and anti-inflammatory potentials in a cell system and in aged rats. Here, we report on the suppressive action of kaempferol on NF-κB activation through the activation of NIK/IKK and MAPK pathways during aging.
Materials and Methods
Animals
Specific pathogen-free male Sprague-Dawley rats were obtained from Samtako (Osan, Republic of Korea). Rats were fed a diet of the following composition: 21% soybean protein, 15% sucrose, 43.65% dextrin, 10% corn oil, 0.15% α-methionine, 0.2% choline chloride, 5% salt mix, 2% vitamin mix, and 3% Solka-Floc® (International Fiber Co., North Tonawanda, NY).
Specific pathogen-free male Sprague-Dawley rats at 7 and 20 months of age were used for young and aged rats, respectively. To examine the anti-inflammatory effects of kaempferol in aged rats, we fed the rats two different levels of kaempferol. Kaempferol was ground and mixed with rat chow at 0.01% or 0.02% final concentration and was fed ad libitum to the 20-month-old rats for 10 days. We estimated each animal consumed on average 2 or 4 mg/kg/day by monitoring the amount of chow consumed each day. In addition, we measured the animal's body weight each day. Young and aged rats not fed kaempferol were fed a normal diet and water ad libitum. After feeding for 10 days, the rats were sacrificed by decapitation, and their kidneys were quickly removed. The tissues were immediately frozen in liquid nitrogen and stored at −80°C. Each group contained five rats. We found no gross morphological changes in these kidneys. Although histopathological examination was not done in the present study, these aged rats were raised under the same condition, including soy protein diets, as those used by Iwasaki et al.,23 who found little evidence of renal lesions in aged rats.
In the study, rats were divided into four groups (five rats in each group). Body weights of each group were as follows: 7-month-old rats, 527 ± 10.6 g; 20-month-old rats not fed kaempferol, 652 ± 30.1 g; 20-month-old rats fed kaempferol (2 mg/kg/day), 650 ± 56.3 g; and 20-month-old rats fed kaempferol (4 mg/kg/day), 704 ± 23.3 g.
Reagents
Kaempferol and tert-butylhydroperoxide (t-BHP) were purchased from Sigma (St. Louis, MO). 2′,7′-Dichlorofluorescin diacetate (DCF-DA) was obtained from Molecular Probes, Inc. (Eugene, OR). Various primary antibodies and a horseradish peroxide-conjugated goat anti-rabbit antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The radionucleotide [32P]ATP and the enhanced chemiluminescence kit were obtained from Amersham (Little Chalfont, UK). Transfection reagent FuGENE® 6 was purchased from Roche (Indianapolis, IN). The Steady-Glo® luciferase assay system was purchased from Promega (Madison, WI). All other chemicals, including the protein assay kit with bicinchoninic acid, were of the highest purity available from Sigma.
Preparation of cytosolic and nuclear fractions from tissue
Three hundred milligrams of frozen kidney tissue was washed with phosphate-buffered saline and homogenized in 2 mL of hypotonic lysis buffer (buffer A) (10 mM HEPES [pH 7.8], 10 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol, 0.1 mM EDTA, and 0.1 mM phenylmethylsulfonyl fluoride) using a tissue homogenizer for 20 seconds. Homogenates were kept on ice for 15 minutes, 125 μL of 10% Nonidet P-40 solution was added and mixed for 15 seconds, and the mixture was centrifuged for 30 seconds at 12,000 g. The pelleted nuclei was washed once with 400 μL of buffer A plus 25 μL of 10% Nonidet P-40, centrifuged, suspended in 200 μL of buffer C (50 mM HEPES [pH 7.8], 50 mM KCl, 300 mM NaCl, 0.1 mM phenylmethylsulfonyl fluoride, and 10% [vol/vol] glycerol), mixed for 20 minutes, and centrifuged for 5 minutes at 12,000 g. The supernatant containing nuclear proteins was harvested, its protein concentration was determined, and it was then stored at −80°C.
Assessments of intracellular oxidative stress in cultured cells
YPEN-1 endothelial cells were purchased from American Type Culture Collection (Rockville, MD). YPEN-1 cells were cultured in Dulbecco's Modified Eagle Medium (Nissui Co., Tokyo, Japan) supplemented with 5% heat-inactivated (56°C for 30 minutes) fetal bovine serum (Gibco, Grand Island, NY).
To determine intracellular reactive species (RS) scavenging activity,22 YPEN-1 cells were seeded in a 96-well plate. After 1 day, the medium was changed to a fresh, serum-free medium. The cells were treated with or without kaempferol for 1 hour. Cells were treated with t-BHP (200 μM) to induce RS generation for 6 hours, and the medium was replaced by a fresh, serum-free medium containing H2DCF-DA (final concentration, 20 μΜ). The fluorescence intensity of 2′,7′-dichlorofluorescin was measured every 5 minutes for 30 minutes using the microplate fluorescence reader with excitation and emission wavelengths of 485 and 535 nm, respectively. For visible detection of intracellular RS, cells were seeded in a six-well plate. After incubation cells with kaempferol and t-BHP, the medium was removed, and DCF-DA (5 μM) was added to cells. YPEN-1 cells were observed using an Axiovert 100 fluorescence microscope (Zeiss, Oberkochen, Germany) at × 120 magnifications.
Assessment of reduced glutathione (GSH)/glutathione disulfide (GSSG) in aged rat kidney
To measure GSH/GSSG levels, 1 mM EDTA/50 mM phosphate buffer was added to the supernatant of trichloroacetic acid-treated homogenates. For the GSH assay, o-phthaldehyde was added to the supernatant of trichloroacetic acid-treated homogenates and incubated for 25 minutes at room temperature. For the GSSG assay, N-ethylmaleimide was added to the supernatant of trichloroacetic acid-treated homogenates and incubated for 30 minutes at room temperature. NaOH (0.5 N) and o-phthaldehyde were added to the sample and incubated for 25 minutes. Both GSH and GSSG levels were measured at excitation and emission wavelengths set at 360 and 460 nm, respectively.24
NF-κB-dependent reporter gene assay
NF-κB activity was examined using a luciferase plasmid DNA, pTAL-NF-κB, that contains a specific binding sequence for NF-κB (BD Biosciences Clontech, Mountain-view, CA).25 Transfection was carried out using FuGENE 6 reagent. In brief, 2 × 104 YPEN-1 cells per well were seeded in 48-well plates. When cultured cells reached about 50% confluence, cells were treated with 0.1 μg of DNA/0.2 μL of FuGENE 6 complexes in a total volume of 500 μL of 5% serum in normal medium for 48 hours. Subsequently, 2.5 μM t-BHP was added to the plate after the medium was changed to serum-free medium, and several doses of kaempferol were incubated for 1 hour. After additional incubation for 6 hours, cells were washed with phosphate-buffered saline, and the Steady-Glo Luciferase luciferase assay system was added to the plate. Luciferase activity was measured by a luminometer (GeNios, Tecan Instruments, Maennedorf, Switzerland).
Western blot analysis
The prepared samples in gel buffer (pH 6.8) (12.5 mM Tris, 4% sodium dodecyl sulfate, 20% glycerol, 10% 2-mer-captoethanol, and 0.2% bromophenol blue) at a ratio of 1:1 were boiled for 5 minutes. Equal amounts of proteins for each sample were separated on 8–17% sodium dodecyl sulfate-polyacryamide minigels at 100 V and transferred to a polyvinylidene difluoride membrane at 100 V for 90 minutes in a wet transfer system (Bio-Rad, Hercules, CA). The membrane was immediately placed into a blocking solution (5% [wt/vol] skim milk powder in Tris-buffered saline-Tween 20 (TBS-T) buffer containing 10 mM Tris, 100 mM NaCl, and 0.1 mM Tween-20, pH 7.4) at room temperature for 1 hour. The membrane was washed in TBS-T buffer for 30 minutes and incubated with a primary antibody at room temperature for 3 hours. After three 10-minute washings in TBS-T buffer, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 2 hours. After three 10-minute washings in TBS-T buffer, antibody labeling was detected using enhanced chemiluminescence and exposed to radiographic film.
Electrophoretic mobility shift assay (EMSA)
The EMSA method was used to characterize the binding activities of NF-κB transcription factors in nuclear extracts.26 The NF-κB oligonucleotide sequence was 5′-GAGAGGCAAGGGGATTCCCTTAGTTAGGA-3′. Protein-DNA binding assays were performed with 20 μg of nuclear protein. Unspecific binding was blocked by using 1 μg of poly(dI-dC) poly(dI-dC). The binding medium contained 5% glycerol, 1% Nonidet P-40, 1 mM MgCl2, 50 mM NaCl, 0.5 mM EDTA, 2 mM dithiothreitol, and 10 mM Tris-HCl, pH 7.5. In each reaction, 20,000 cpm of a radiolabeled probe was included. Samples were incubated at room temperature for 20 minutes, and the nuclear protein with 32P-labeled oligonucleotide complex was separated from free 32P-labeled oligonucleotide by electrophoresis through a 5% native polyacrylamide gel in a running buffer containing 50 mM Tris (pH 8.0), 45 mM borate, and 0.5 mM EDTA. After separation was achieved, the gel was vacuum-dried for autoradiography and exposed to Fuji (Tokyo) X-ray film for 1–2 days at −80°C.
Statistical analysis
Results are presented as mean ± standard error of three individual experiments; each measurement was performed in triplicate. Statistical significance was tested using the one-factor analysis of variance test, and, if significant, the Newman-Keuls test was applied. Values of P < .05 were considered statistically significant.
Results
Inhibitory effect of kaempferol on t-BHP-induced oxidative stress
The oxidant t-BHP is well known to induce oxidative stress27 and was used as a positive control. We have determined the effects of kaempferol on t-BHP-induced intra-cellular RS generation in YPEN-1 cells using DCF-DA, which is oxidized by RS to fluorescent 2′,7′-dichlorofluorescin. YPEN-1 cells treated with 200 μM t-BHP displayed fluorescence intensity before incorporation with DCF-DA. Intracellular RS formation resulting from t-BHP treatment was significantly reduced when kaempferol (0.008, 0.2, or 5 μM) was present in the medium (Fig. 1A). Data on kaempferol concentrations as low as 8 nM showed its antioxidative efficacy in YPEN-1 cell (Fig. 1A).
FIG. 1.
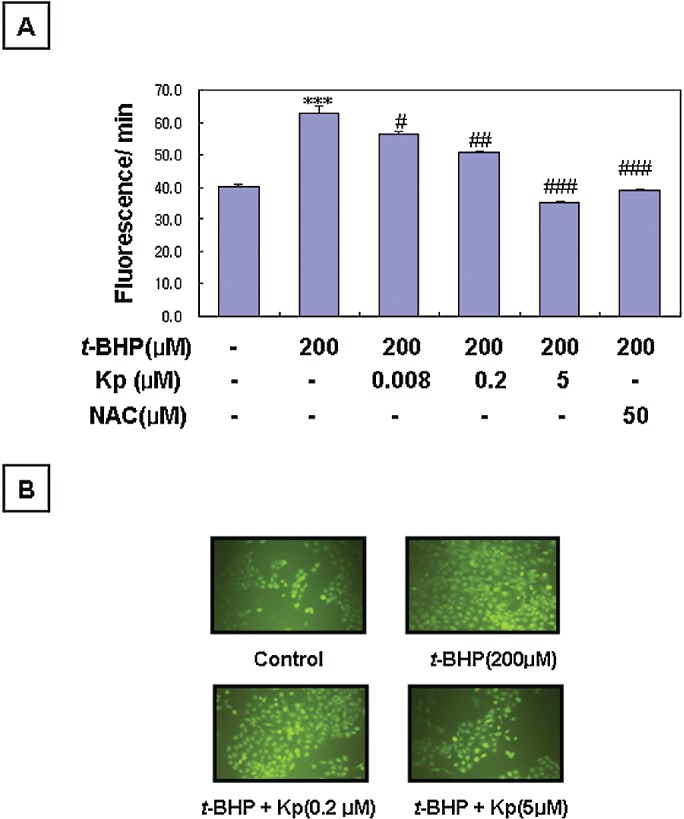
t-BHP-induced RS generation was suppressed by kaempferol (Kp) in YPEN-1 cells. (A) Quantitative analysis of fluorescence intensity using DCF-DA was detected after treatment with vehicle or 200 μM t-BHP in the absence or presence of 0.008, 0.2, and 5 μM Kp and 50 μM N-acetyllysine (NAC) for 6 hours. Results of one-factor analysis of variance: ***P < .001 versus untreated control; #P < .05, ##P < .01, ###P < .001 versus 200 μM t-BHP-treated group. (B) Intracellular RS were detected by DCF-DA using a fluorescence microscope: untreated control, 200 μM t-BHP treated alone, 200 μM t-BHP and 0.2 μM Kp, and 200 μM t-BHP and 5 μM Kp.
As shown in Figure 1B, fluorescence microscope observations showed kaempferol-treated cells exhibited lower fluorescence intensity. Thus, the results showed that kaempferol has an intracellular capacity to prevent oxidative stress.
Modulation of redox status by kaempferol in aged rat kidney
To follow up on our in vitro observations, we tested the effect of kaempferol in the modulation of redox status in aged animal models. RS generation and the GSH/GSSG ratio were measured in aged rat kidney. As shown in Figure 2A, RS generation in the aged kaempferol-supplemented rats was lower than that of their aged unsupplemented counterparts. A decrease of the oxidative defense system was further confirmed by changes in GSH/GSSG ratio in aged kidney homogenates. The GSH/GSSG ratio (Fig. 2B) in the young groups was higher than those of aged groups, while aged rats fed kaempferol had a higher ratio than aged rats not fed kaempferol. These results indicate that kaempferol aided the system to recover an elevated oxidative status during aging.
FIG. 2.
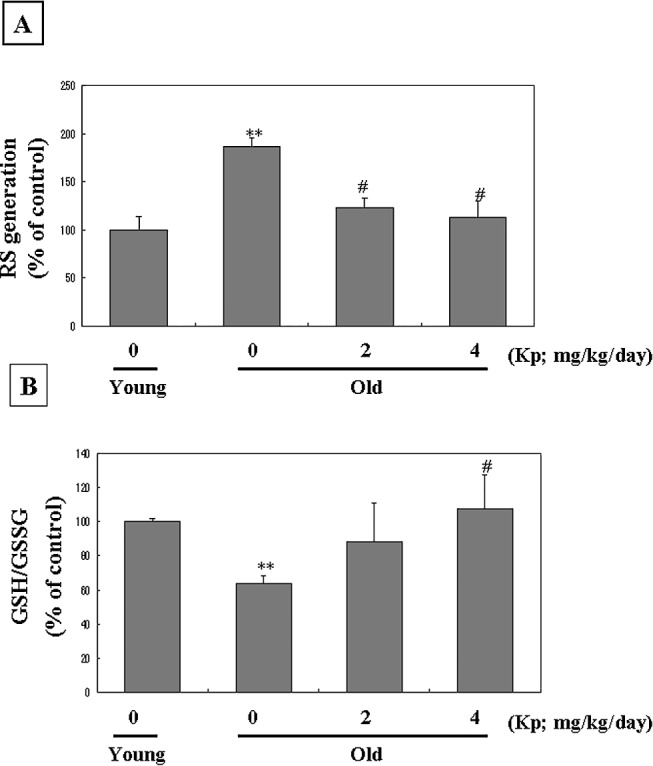
Kaempferol (Kp) modulated redox imbalance in aged rats. (A) The DCF-DA method was used to determine the effect of Kp on RS generation in aged kidney homogenate. (B) GSH/GSSG ratio in kidney homogenates. Young (7 months of age) and aged (20 months of age) rats were utilized. Kp (2 or 4 mg/kg/day) was fed to the aged group for 10 days. Data are mean ± SE values of five rats in each group. Results of one-factor analysis of variance: **P < .01 versus young rats; #P < .05 versus aged rats not fed Kp.
Suppression of NF-κB activation by kaempferol in aged rat kidney and YPEN-1 cells
To determine whether or not kaempferol has an effect upon increased NF-κB activation in aged kidney, we first examined nuclear protein levels by western blot using p65-and p50-specific polyclonal antibodies. Data in Figure 3A clearly reveal that the nuclear translocation of NF-κB significantly increased in aged rat kidney and that aged rats fed kaempferol showed dose-dependently decreased NF-κB activity compared to their aged non–kaempferol-fed counterparts. The levels of phospho-IκBα proteins in cytoplasmic extract increased in the aged rats, but aged kaempferol-fed rats showed lower levels of these proteins (Fig. 3A). Second, to verify DNA binding of NF-κB, EMSA was carried out with nuclear proteins isolated from young, aged, and kaempferol-fed aged. Results shown in Figure 3B indicate that the binding activity of NF-κB was up-regulated during aging, while kaempferol suppressed NF-κB activity.
FIG. 3.
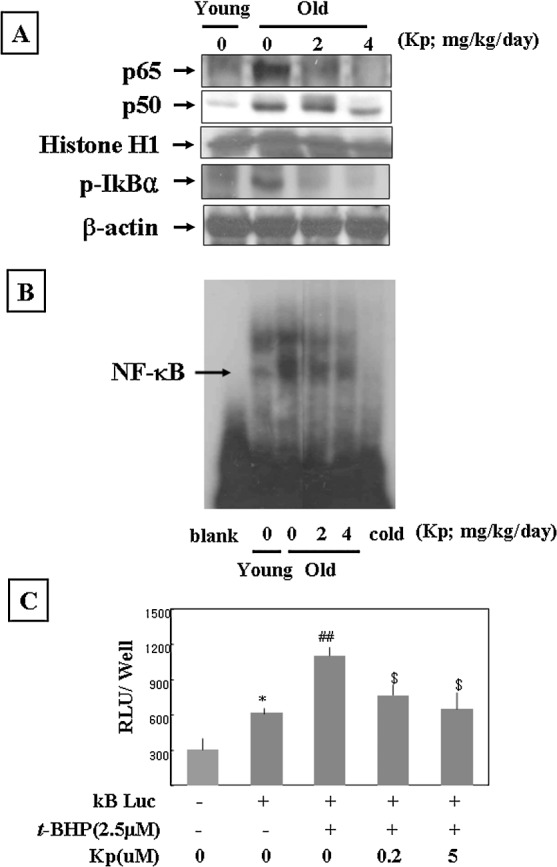
Kaempferol (Kp) suppressed the increase of NF-κB activity in aged rats and YPEN-1 cells. (A) Western blot was performed to detect nuclear p50, p65, and phospho-lκBα protein levels in kidney homogenates (30 μg of protein) from young, aged, or Kp-fed aged rats. Levels were normalized to histone H1 and β-actin. One representative blot of p50, p65, and phospho-IκBα is shown from three experiments that yielded similar results. (B) The EMSA method was used to compare NF-κB binding activities between aged rats fed Kp and their counterparts. Blank, probe without nuclear protein sample; cold, competition assay using a 100-fold excess of an unlabeled NF-κB oligonucleotide. One representative result is shown from three experiments that yielded similar results. (C) YPEN-1 cells were transiently transfected with an NF-κB-containing plasmid linked to the luciferase (Luc) gene. The cells were co-treated with 2.5 μM t-BHP and 0.2 μM or 5 μM Kp for 6 hours. Luc activity is presented as a percentage of activity compared with control cells. Statistical significance was calculated of differences between untreated control and treated groups in Luc activity of NF-κB. Results of one-factor analysis of variance: *P < .05 versus non-transfection control; ##P < .001 versus transfected and untreated t-BHP; $P < .05 versus transfected with 2.5 μM t-BHP, respectively. RLU, relative luminescence units.
To verify the effect of kaempferol on oxidative stress-induced NF-κB activation in endothelial cells, luciferase activity was examined using t-BHP treatment in the absence or presence of kaempferol after transient transfection of a plasmid containing the NF-κB consensus sequence and luciferase reporter (Fig. 3C). NF-κB luciferase activity increased by twofold in 2.5 μM t-BHP after a 6-hour incubation, and kaempferol treatment gave dose-dependent suppression of NF-κB luciferase activity. Overall, the results indicate that the activation of NF-κB by t-BHP-induced oxidative stress was inhibited by kaempferol.
Inhibition of NF-κB-responsive genes expression by kaempferol in aged rat kidney
The expressions of NF-κB-dependent genes, namely, COX-2, inducible nitric oxide synthase (iNOS), monocyte chemoattractant protein-1 (MCP-1), and regulated upon activation, and normal T-cell expressed and secreted (RANTES), were examined in aged rat kidney. These genes have been known to have NF-κB binding sites at their promoter regions and to be controlled by NF-κB regulation.28–31 As shown in Figure 4, kaempferol reduced COX-2, iNOS, MCP-1, and RANTES protein levels, which were increased with age.
FIG. 4.
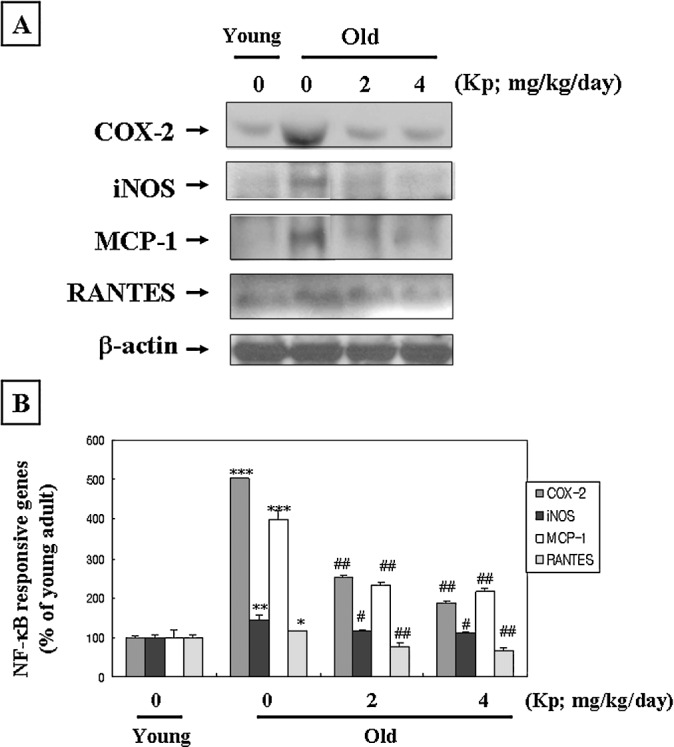
Kaempferol (Kp) modulated NF-κB-dependent genes in aged rats. (A) Western blot analysis was performed to detect COX-2, iNOS, MCP-1, and RANTES protein levels in cytoplasmic fractions (80 μg of protein) from each group. One representative blot of each protein from each group is shown from three experiments that yielded similar results. (B) The level of protein was quantified by densitometry as a percentage of the level of the young rats. Data are mean ± SE values from three individual experiments. Results of one-factor analysis of variance: *P < .05, **P < .01, ***P < .001 versus young rats; #P < .05, ##P < .01 versus aged rats not fed Kp.
Kaempferol suppressed NF-κB via NIK/IKK and MAPK pathways in aged rat kidney
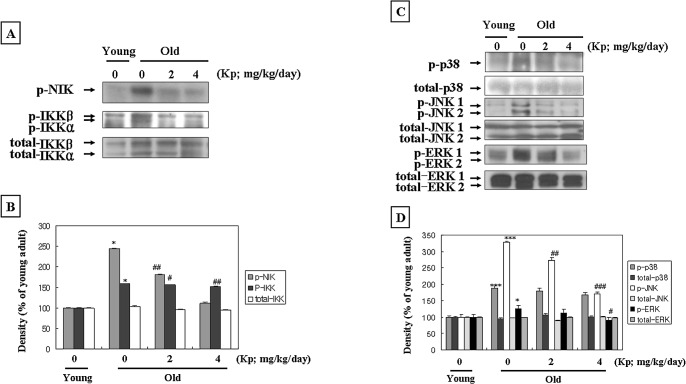
We tested the effect of kaempferol on the phosphorylation of NIK/IKK and MAPKs, which are upstream of NF-κB. Phosphorylation of NIK/IKK (Fig. 5A) and MAPKs (Fig. 5C) significantly increased in aged rat kidney. However, the aged kaempferol-fed rats showed lower, dose-dependent phosphorylation levels. Kaempferol supplementation significantly inhibited oxidative stress-induced phosphorylation of NIK/IKK and MAPKs during aging.
FIG. 5.
Kaempferol (Kp) inhibited the NIK/IKK and MAPKs pathways. (A) Western blot analysis was performed to detect phospho-NIK, phospho-IKKα/β, and total-IKKα/β in kidney cytoplasmic extracts (80 μg of protein) from each group. Levels were normalized to total-IKKα/β. (B) The level of protein was quantified by densitometry as a percentage of the level of the young rats. Results of one-factor analysis of variance: *P < .05 versus young rats; #P < .05, ##P < .01 versus aged rats not fed Kp. (C) Phospho-38, phospho-c-jun N-terminal kinase (JNK)1/2, and phospho-extracellular signal-regulated kinase (ERK)1/2 protein levels in cytoplasmic fractions (80 μg of protein) from each group. Levels were normalized to total-p38, total JNK1/2, and total-ERK1/2. One representative blot of each protein from each group is shown from three experiments that yielded similar results. (D) The level of protein was quantified by densitometry as a percentage of the level of young rats. Results of one-factor analysis of variance: *P < .05, ***P < .001 versus young rats; #P < .05, ##P < .01, ###P < .001 versus aged rats not fed Kp.
Discussion
Kaempferol, a phytoestrogen, has a flavonoid structure. It is an important constituent of the human diet, protecting against various oxidative stresses and inflammatory-related diseases such as age-related chronic disorders.2,3
Although several researchers have reported on kaempferol's antioxidative action, no reports show the effects of short-term supplemental dietary kaempferol on the modulation of NF-κB activation and its upstream signal pathway through redox maintenance in aged rat kidney. In the present study, we examined the in vitro antioxidant activities of kaempferol and its anti-inflammatory effects on NF-κB activity in aged rats based on the in vitro results.
Many lines of evidence have shown that oxidative stress plays an important role in the pathogenesis of many diseases, including inflammatory diseases.32 The kidney is an especially vulnerable organ to oxidative stress during aging, as shown by oxidant-induced nephritis, vasculitis, toxic nephropathies, pyelonephritis, and acute renal failure.21,33,34 These diseases are likely to be mediated in part by age-related oxidative insults due to redox imbalance. Therefore, researchers have focused on development of safe and effective antioxidants from natural phytochemicals. Of the many effective antioxidants, phytoestrogens, including kaempferol, have been reported to possess free radical scavenging properties.2,3 In the present study, we also were able to confirm the anti-oxidative property of kaempferol as a redox modulator in vitro and in vivo. Of the four phytoestrogens tested (genistein, daidzein, biochanin A, and kaempferol), kaempferol showed the strongest antiradical properties, with 50% inhibitory activity of RS and ONOO−. Kaempferol scavenged the reactivity of RS and ONOO− 10-fold greater than genistein, daidzein, and biochanin A (data not shown).
We next examined anti-oxidative effects of kaempferol on t-BHP-induced cellular oxidative stress in YPEN-1 cells (Fig. 1). Our data show that treatment with kaempferol inhibited t-BHP-induced cellular oxidative status. Therefore, based on our in vitro results, we carried out an in vivo experiment to verify the anti-inflammatory effects of kaempferol on NF-κB, a key transcription factor in the inflammatory process.
Under the condition of a cumulative oxidative stress-induced redox imbalance, as would occur with the aging process, activated NF-κB is translocated from the cytoplasm into the nucleus. A central mechanism of regulation is the interaction of the NF-κB/Rel proteins with specific inhibitory proteins called IκBs. Activation by stimuli requires sequential phosphorylation of IκB by IKK, ubiquitination, and degradation by the proteasome pathway.20 NF-κB rapidly modulates the expression of inflammatory gene products, including cytokines, COX-2, and iNOS.28–31
We elucidated the anti-inflammatory effects of kaempferol on NF-κB activity and its related gene expressions in the presence of oxidative stress in aged kidney. Our data show that treatment with kaempferol inhibited accumulated oxidative stress and restored the GSH/GSSG ratio (Fig. 2). The result indicates that in aged rats, kaempferol modulated redox status and had a potent antioxidative capacity. Moreover, based on our results using western blot, EMSA, and the reporter assay, we conclude that kaempferol inhibited proteolytic degradation of IκB, binding of the p50/p65 heterodimer, and NF-κB-dependent gene expressions in aged rat kidney (Figs. 3 and 4).
NF-κB activation is mediated by two, distinctly different redox-related signaling pathways. First, the NIK/IKK pathway is involved in the induction of transcriptional activation of NF-κB.35 Second, MAPKs regulate NF-κB activation via multiple mechanisms.17,18 Previously, our group reported that NIK/IKK, MAPKs, and NF-κB have increased activity during aging10 and that diverse anti-inflammatory compounds suppress pro-inflammatory processes.36 Our data from this current study also show that kaempferol significantly suppressed the NIK/IKK and MAPK pathways that lead to NF-κB activation in aged kidney tissues (Fig. 5).
The present study documented that kaempferol restored redox imbalance through its efficient RS scavenging capacity and modulated pro-inflammatory NF-κB activation via the NIK/IKK and MAPK pathways in aging. These studies demonstrate that kaempferol is an efficient anti-inflammatory compound with the ability to attenuate oxidative stress-induced inflammation in aged rat kidney.
Acknowledgments
This work was supported by Korea Science and Engineering Foundation (KOSEF) grants funded by the Korean government (MOST) (numbers 2007-00376 and R01-2005-000-10661-0) and by the Technology Development Program (No. 105075 3) for Agriculture and Forestry, Ministry for Agriculture, Forestry and Fisheries. We also thank the “Aging Tissue Bank” for the supply of kidney tissue.
References
- 1. Branca F: Dietary phyto-oestrogens and bone health. Proc Nutr Soc 2003;62:877–887 [DOI] [PubMed] [Google Scholar]
- 2. Bhathena SJ, Velasquez MT: Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr 2002;76:1191–1201 [DOI] [PubMed] [Google Scholar]
- 3. Usui T: Pharmaceutical prospects of phytoestrogens. Endocr J 2006;53:7–20 [DOI] [PubMed] [Google Scholar]
- 4. Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T: Effect of kaempferol on the production and gene expression of monocyte chemoattractant protein-1 in J774.2 macrophages. Pharmacol Rep 2005;57:107–112 [PubMed] [Google Scholar]
- 5. García-Mediavilla V, Crespo I, Collado PS, Esteller A, Sánchez-Campos S, Tuñón MJ, Gonzalez-Gallego J: The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang liver cells. Eur J Pharmacol 2007;557:221–229 [DOI] [PubMed] [Google Scholar]
- 6. Chen CC, Chow MP, Huang WC, Lin YC, Chang YJ: Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: structure-activity relationships. Mol Pharmacol 2004;66:683–693 [DOI] [PubMed] [Google Scholar]
- 7. Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ: Distinctive antioxidant and antiinflammatory effects of flavonols. J Agric Food Chem 2006;54:9798–9804 [DOI] [PubMed] [Google Scholar]
- 8. Yu BP, Chung HY: Adaptive mechanisms to oxidative stress during aging. Mech Ageing Dev 2006;27:436–443 [DOI] [PubMed] [Google Scholar]
- 9. Yu BP: Membrane alteration as a basis of aging and the protective effects of calorie restriction. Mech Ageing Dev 2005;126:1003–1010 [DOI] [PubMed] [Google Scholar]
- 10. Chung HY, Sung B, Jung KJ, Zou Y, Yu BP: The molecular inflammatory process in aging. Antioxid Redox Signal 2006;8:572–581 [DOI] [PubMed] [Google Scholar]
- 11. Chung HY, Cesari M, Anton S, Marzetti F, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C: Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res Rev 2008;8:18–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung HY, Kim HJ, Kim KW, Choi JS, Yu BP: Molecular inflammation hypothesis of aging based on the anti-aging mechanism of calorie restriction. Microsc Res Tech 2002;15:264–272 [DOI] [PubMed] [Google Scholar]
- 13. Hatada EN, Krappmann D, Scheidereit C: NF-κB and the innate immune response. Curr Opin Immunol 2000;12:52–58 [DOI] [PubMed] [Google Scholar]
- 14. Lavrovsky Y, Chatterjee B, Clark RA, Roy AK: Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp Gerontol 2000;35:521–532 [DOI] [PubMed] [Google Scholar]
- 15. Karin M: How NF-kappa B is activated: the role of the IkappaB kinase (IKK) complex. Oncogene 1999;18:6867–6874 [DOI] [PubMed] [Google Scholar]
- 16. Malinin NL, Boldin MP, Kovalenko AV, Wallach D: MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature 1997;385:540–544 [DOI] [PubMed] [Google Scholar]
- 17. Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G: p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J Biol Chem 1998;273:3285–3290 [DOI] [PubMed] [Google Scholar]
- 18. Darieva Z, Lasunskaia EB, Campos MN, Kipnis TL, Da Silva WD: Activation of phosphatidylinositol 3-kinase and c-Jun-N-terminal kinase cascades enhances NF-kappaB-dependent gene transcription in BCG-stimulated macrophages through promotion of p65/p300 binding. J Leukoc Biol 2004;75:689–697 [DOI] [PubMed] [Google Scholar]
- 19. Barnes PJ, Karin M: Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 1997;336:1066–1071 [DOI] [PubMed] [Google Scholar]
- 20. Baldwin AS: The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol 1996;14:681–683 [DOI] [PubMed] [Google Scholar]
- 21. Csiszar A, Toth J, Peti-Peterdi J, Ungvari Z: The aging kidney: role of endothelial oxidative stress and inflammation. Acta Physiol Hung 2007;94:107–115 [DOI] [PubMed] [Google Scholar]
- 22. Go EK, Jung KJ, Kim JY, Yu BP, Chung HY: Betaine suppresses proinflammatory signaling during aging: the involvement of nuclear factor-kappaB via nuclear factor-inducing kinase/IkappaB kinase and mitogen-activated protein kinases. J Gerontol A Biol Sci Med Sci 2005;60:1252–1264 [DOI] [PubMed] [Google Scholar]
- 23. Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP: The influence of dietary protein source on longevity and age-related disease processes of Fischer rats. J Gerontol 1988;43:B5–B12 [DOI] [PubMed] [Google Scholar]
- 24. Hissin PJ, Hilif R: A fluorometric method for the determination of oxidized and reduced glutathione in tissues. Anal Biochem 1976;74:214–226 [DOI] [PubMed] [Google Scholar]
- 25. Altuwaijri S, Lin HK, Chuang KH, Lin WJ, Yeh S, Hanchett LA, Rahman MM, Kang HY, Tsai MY, Zhang Y, Yang L, Chang C: Interruption of nuclear factor kappaB signaling by the androgen receptor facilitates 12-O-tetradecanoylphorbolacetate-induced apoptosis in androgen-sensitive prostate cancer LNCaP cells. Cancer Res 2003;63:7106–7112 [PubMed] [Google Scholar]
- 26. Kerr LD: Electrophoretic mobility shift assay. Methods Enzymol 1995;254:619–632 [DOI] [PubMed] [Google Scholar]
- 27. Thornalley PJ, Trotta RJ, Stern A: Free radical involvement in the oxidative phenomena induced by tert-butyl hydroperoxide in erythrocytes. Biochim Biophys Acta 1983;759:16–22 [DOI] [PubMed] [Google Scholar]
- 28. Murphy WJ, Muroi M, Zhang X, Suzuki T, Russell SW: Both a basal and an enhancer IB element is required for full induction of the mouse inducible nitric oxide synthase gene. J Endotoxin Res 1996;3:381–393 [Google Scholar]
- 29. Ping D, Jones PL, Boss JM: TNF regulates the in vivo occupancy of both distal and proximal regulatory regions of the MCP-1/JE gene. Immunity 1996;4:455–469 [DOI] [PubMed] [Google Scholar]
- 30. Moriuchi H, Moriuchi M, Fauci AS: Nuclear factor-kappa B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J Immunol 1997;158:3483–3491 [PubMed] [Google Scholar]
- 31. Yamamoto K, Arakawa T, Taketani Y, Takahashi Y, Hayashi Y, Ueda N: TNF alpha-dependent induction of cyclooxygenase-2 mediated by NF kappa B and NF-IL6. Adv Exp Med Biol 1997; 407:185–189 [PubMed] [Google Scholar]
- 32. Purushothaman KR, Meerarani P, Moreno PR: Inflammation and neovascularization in diabetic atherosclerosis. Indian J Exp Biol 2007;45:93–102 [PubMed] [Google Scholar]
- 33. Ruiz-Torres P, Gonzalez-Rubio M, Lucio-Cazaña FJ, Ruiz-Villaespesa A, Rodriguez-Puyol M, Rodriguez-Puyol D: Reactive oxygen species and platelet-activating factor synthesis in age-related glomerulosclerosis. J Lab Clin Med 1994;124:489–495 [PubMed] [Google Scholar]
- 34. Baud L, Ardaillou R: Involvement of reactive oxygen species in kidney damage. Br Med Bull 1993;49:621–629 [DOI] [PubMed] [Google Scholar]
- 35. Je JH, Lee JY, Jung KJ, Sung B, Go EK, Yu BP, Chung HY: NF-kappaB activation mechanism of 4-hydroxyhexenal via NIK/IKK and p38 MAPK pathway. FEBS Lett 2004;21:183–189 [DOI] [PubMed] [Google Scholar]
- 36. Kim JY, Jung KJ, Choi JS, Chung HY: Modulation of the age-related nuclear factor-kappaB (NF-kappaB) pathway by hesperetin. Aging Cell 2006;5:401–411 [DOI] [PubMed] [Google Scholar]