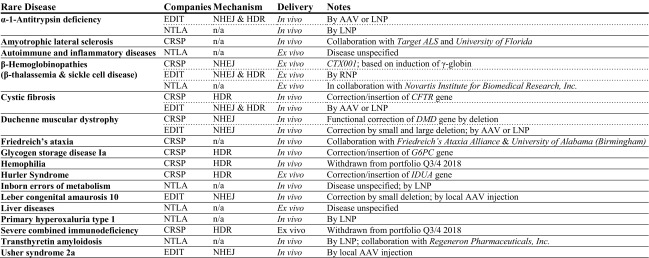
Table 3.
Rare disease product pipelines of leading companies based on clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) technology
Only product development for non-malignant diseases is listed, sorted by rare disease. Information has been extracted from the product pipelines given on the company websites of CRISPR Therapeutics [138], Editas Medicine [139], and Intellia Therapeutics [140], last update 22 November 2018. Recent changes in the portfolio, as indicated, suggest ongoing readjustments of early-stage targets, which may also affect current portfolio content or result in additional disease targets in the future
AAV adeno-associated virus, CRSP CRISPR Therapeutics, EDIT Editas Medicine, HDR homology-directed repair, LNP lipid nanoparticle, n/a not available, NHEJ non-homologous end-joining, NTLA Intellia Therapeutics, RNP ribonucleoprotein particle