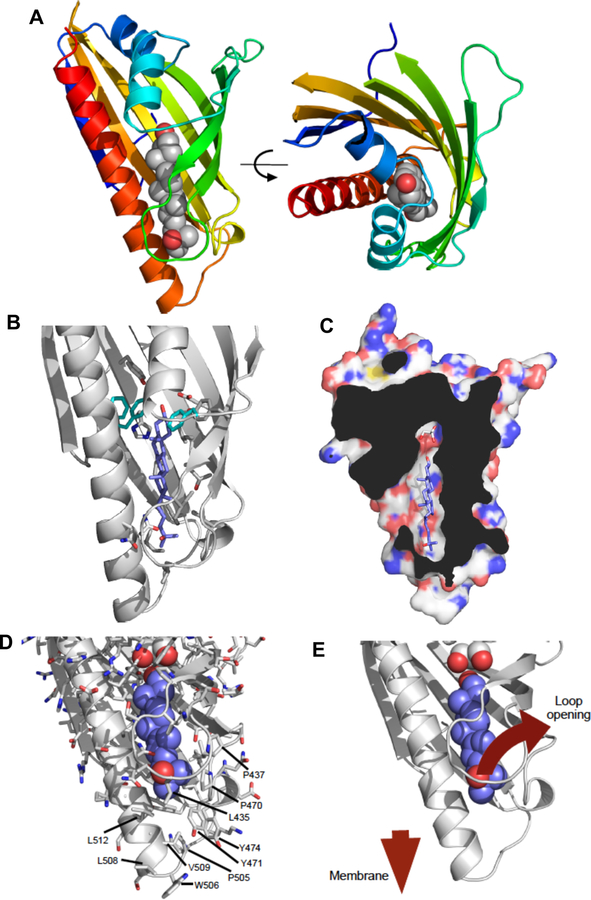
Figure 2. Crystal structure of the sterol-binding domain of Aster-A.
(A) The crystal structure of the ASTER domain of the mouse Aster-A. The ribbon representation is colored from blue-red amino-carboxy terminus. The 25-hydroxycholesterol ligand is shown as atomic spheres: grey-carbon, red-oxygen. The right-hand panel is rotated 90°about the indicated axis. The ligand-binding pocket is situated between a concave beta-sheet and a long carboxy-terminal helix.
(B) Details of the 25-hydroxycholesterol ligand-binding pocket. The left-hand panel shows key sidechains within the ligand pocket that mediate interaction with the ligand. Phe405, Tyr524 and Phe525 (cyan) seem to determine the orientation of the ligand and are markedly different in character from the equivalent residues in the yeast Lam proteins (see also Supplemental Figure 3).
(C) Cut-away view of the surface of mouse Aster A showing the ligand-binding pocket. The ligand is completely enclosed with the exception of an opening towards the left of the pocket. The pocket is significantly larger than the ligand beyond the C3-OH group. This additional space is occupied by a glycerol molecule.
(D-E) Potential mechanism for loading cholesterol into the ASTER domain. Structural rearrangements would be essential for cholesterol to gain access to the binding pocket. This is likely to involve the loop comprising amino acids 430–439 that wraps around the ligand. The surface of this region of the ASTER domain is relatively non-polar in character (see labeled amino acids), but with a number of prominent basic residues. It seems likely that this region of the protein will come into contact with the negatively charged / non-polar lipid bilayer into order to facilitate both loading and unloading of the cholesterol ligand. See also Supplemental Figures 2 and 3.